Clustering of syndecan-4 and integrin beta1 by laminin alpha 3 chain-derived peptide promotes keratinocyte migration
- PMID: 19403697
- PMCID: PMC2704153
- DOI: 10.1091/mbc.e08-09-0977
Clustering of syndecan-4 and integrin beta1 by laminin alpha 3 chain-derived peptide promotes keratinocyte migration
Abstract
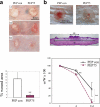
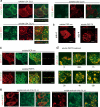
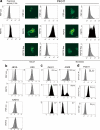
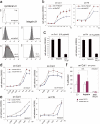
Syndecans function as receptors for extracellular matrix (ECM) with integrins in cell spreading. However, the molecular mechanism of their specific involvement in cell migration or in wound healing has not been elucidated yet. Here, we report that a synthetic peptide, PEP75, which contains the syndecan-binding sequence of the laminin alpha 3LG4 module, induces keratinocyte migration in in vitro and in vivo. Soluble PEP75 induced the clustering of syndecan-4 and conformation-modified integrin beta1 colocalized with syndecan-4 in soluble PEP75-induced clusters. Treatment of cells in solution with PEP75 resulted in the exposure of the P4G11 antibody epitope of integrin beta1 in immunostaining as well as in flow cytometry and augmented integrin beta1-dependent cell adhesion to ECM. Pulldown assays demonstrated that PEP75 bound to syndecan-4, but not to integrin beta1. A siRNA study revealed a role for syndecan-4 in PEP75-induced up-regulation of P4G11 antibody binding and migration of HaCaT cells. We conclude that binding of soluble PEP75 to syndecan-4 induces the coupling of integrin beta1, which is associated with integrin beta1-conformational changes and activation, and leads to keratinocyte migration. To activate integrin function through syndecans could be a novel therapeutic approach for chronic wound.
Figures



References
-
- Alexopoulou A. N., Multhaupt H. A., Couchman J. R. Syndecans in wound healing, inflammation and vascular biology. Int. J. Biochem. Cell Biol. 2007;39:505–528. - PubMed
-
- Berrier A. L., Yamada K. M. Cell-matrix adhesion. J. Cell Physiol. 2007;213:565–573. - PubMed
-
- Carey D. J., Stahl R. C., Tucker B., Bendt K. A., Cizmeci-Smith G. Aggregation-induced association of syndecan-1 with microfilaments mediated by the cytoplasmic domain. Exp. Cell Res. 1994;214:12–21. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

