A versatile zero background T-vector system for gene cloning and functional genomics
- PMID: 19403729
- PMCID: PMC2705043
- DOI: 10.1104/pp.109.137125
A versatile zero background T-vector system for gene cloning and functional genomics
Abstract
With the recent availability of complete genomic sequences of many organisms, high-throughput and cost-efficient systems for gene cloning and functional analysis are in great demand. Although site-specific recombination-based cloning systems, such as Gateway cloning technology, are extremely useful for efficient transfer of DNA fragments into multiple destination vectors, the two-step cloning process is time consuming and expensive. Here, we report a zero background TA cloning system that provides simple and high-efficiency direct cloning of PCR-amplified DNA fragments with almost no self-ligation. The improved T-vector system takes advantage of the restriction enzyme XcmI to generate a T-overhang after digestion and the negative selection marker gene ccdB to eliminate the self-ligation background after transformation. We demonstrate the feasibility and flexibility of the technology by developing a set of transient and stable transformation vectors for constitutive gene expression, gene silencing, protein tagging, protein subcellular localization detection, and promoter fragment activity analysis in plants. Because the system can be easily adapted for developing specialized expression vectors for other organisms, zero background TA provides a general, cost-efficient, and high-throughput platform that complements the Gateway cloning system for gene cloning and functional genomics.
Figures
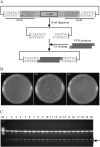


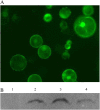


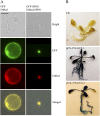
References
-
- Bernard P, Couturier M (1992) Cell killing by the F plasmid CcdB protein involves poisoning of DNA-topoisomerase II complexes. J Mol Biol 226 735–745 - PubMed
-
- Chen QJ, Zhou HM, Chen J, Wang XC (2006. a) Using a modified TA cloning method to create entry clones. Anal Biochem 358 120–125 - PubMed
-
- Chen S, Tao L, Zeng L, Vega-Sanchez ME, Umemura K, Wang GL (2006. b) A highly efficient transient protoplast system for analyzing defence gene expression and protein-protein interactions in rice. Mol Plant Pathol 7 417–427 - PubMed
-
- Christensen AH, Sharrock RA, Quail PH (1992) Maize polyubiquitin genes: structure, thermal perturbation of expression and transcript splicing, and promoter activity following transfer to protoplasts by electroporation. Plant Mol Biol 18 675–689 - PubMed
-
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16 735–743 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources

