Activity-dependent repression of Cbln1 expression: mechanism for developmental and homeostatic regulation of synapses in the cerebellum
- PMID: 19403810
- PMCID: PMC6665857
- DOI: 10.1523/JNEUROSCI.4473-08.2009
Activity-dependent repression of Cbln1 expression: mechanism for developmental and homeostatic regulation of synapses in the cerebellum
Abstract
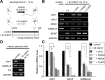
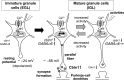
Cbln1, which belongs to the C1q/tumor necrosis factor superfamily, is released from cerebellar granule cells and plays a crucial role in forming and maintaining excitatory synapses between parallel fibers (PFs; axons of granule cells) and Purkinje cells not only during development but also in the adult cerebellum. Although neuronal activity is known to cause morphological changes at synapses, how Cbln1 signaling is affected by neuronal activity remains unclear. Here, we show that chronic stimulation of neuronal activity by elevating extracellular K(+) levels or by adding kainate decreased the expression of cbln1 mRNA within several hours in mature granule cells in a manner dependent on L-type voltage-dependent Ca(2+) channels and calcineurin. Chronic activity also reduced Cbln1 protein levels within a few days, during which time the number of excitatory synapses on Purkinje cell dendrites was reduced; this activity-induced reduction of synapses was prevented by the addition of exogenous Cbln1 to the culture medium. Therefore, the activity-dependent downregulation of cbln1 may serve as a new presynaptic mechanism by which PF-Purkinje cell synapses adapt to chronically elevated activity, thereby maintaining homeostasis. In addition, the expression of cbln1 mRNA was prevented when immature granule cells were maintained in high-K(+) medium. Since immature granule cells are chronically depolarized before migrating to the internal granule layer, this depolarization-dependent regulation of cbln1 mRNA expression may also serve as a developmental switch to facilitate PF synapse formation in mature granule cells in the internal granule layer.
Figures






References
-
- Bading H, Ginty DD, Greenberg ME. Regulation of gene expression in hippocampal neurons by distinct calcium signaling pathways. Science. 1993;260:181–186. - PubMed
-
- Bao D, Pang Z, Morgan JI. The structure and proteolytic processing of Cbln1 complexes. J Neurochem. 2005;95:618–629. - PubMed
-
- Bessho Y, Nakanishi S, Nawa H. Glutamate receptor agonists enhance the expression of BDNF mRNA in cultured cerebellar granule cells. Brain Res Mol Brain Res. 1993;18:201–208. - PubMed
-
- Bessho Y, Nawa H, Nakanishi S. Selective up-regulation of an NMDA receptor subunit mRNA in cultured cerebellar granule cells by K(+)-induced depolarization and NMDA treatment. Neuron. 1994;12:87–95. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
