Delta-catenin regulates spine and synapse morphogenesis and function in hippocampal neurons during development
- PMID: 19403811
- PMCID: PMC2763482
- DOI: 10.1523/JNEUROSCI.0835-09.2009
Delta-catenin regulates spine and synapse morphogenesis and function in hippocampal neurons during development
Abstract
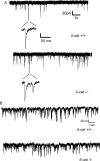
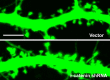
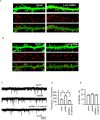
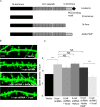
The maintenance of spine and synapse number during development is critical for neuronal circuit formation and function. Here we show that delta-catenin, a component of the cadherin-catenin cell adhesion complex, regulates spine and synapse morphogenesis during development. Genetic ablation or acute knockdown of delta-catenin leads to increases in spine and synapse density, accompanied by a decrease in tetrodotoxin induced spine plasticity. Our results indicate that delta-catenin may mediate conversion of activity-dependent signals to morphological spine plasticity. The functional role of delta-catenin in regulating spine density does not require binding to cadherins, but does require interactions with PDZ domain-containing proteins. We propose that the perturbations in spine and synaptic structure and function observed after depletion of delta-catenin during development may contribute to functional alterations in neural circuitry, the cognitive deficits observed in mutant mice, and the mental retardation pathology of Cri-du-chat syndrome.
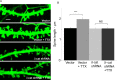
Figures



Similar articles
-
δ-Catenin Regulates Spine Architecture via Cadherin and PDZ-dependent Interactions.J Biol Chem. 2015 Apr 24;290(17):10947-57. doi: 10.1074/jbc.M114.632679. Epub 2015 Feb 27. J Biol Chem. 2015. PMID: 25724647 Free PMC article.
-
p120 catenin regulates dendritic spine and synapse development through Rho-family GTPases and cadherins.Neuron. 2006 Jul 6;51(1):43-56. doi: 10.1016/j.neuron.2006.05.018. Neuron. 2006. PMID: 16815331 Free PMC article.
-
Cadherins and catenins in dendrite and synapse morphogenesis.Cell Adh Migr. 2015;9(3):202-13. doi: 10.4161/19336918.2014.994919. Cell Adh Migr. 2015. PMID: 25914083 Free PMC article. Review.
-
Deletion of the neuron-specific protein delta-catenin leads to severe cognitive and synaptic dysfunction.Curr Biol. 2004 Sep 21;14(18):1657-63. doi: 10.1016/j.cub.2004.08.065. Curr Biol. 2004. PMID: 15380068
-
Synaptic contact dynamics controlled by cadherin and catenins.Trends Cell Biol. 2005 Apr;15(4):216-21. doi: 10.1016/j.tcb.2005.02.002. Trends Cell Biol. 2005. PMID: 15817378 Review.
Cited by
-
Arvcf Dependent Adherens Junction Stability is Required to Prevent Age-Related Cortical Cataracts.Front Cell Dev Biol. 2022 Jul 6;10:840129. doi: 10.3389/fcell.2022.840129. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35874813 Free PMC article.
-
p120-catenin regulates REST and CoREST, and modulates mouse embryonic stem cell differentiation.J Cell Sci. 2014 Sep 15;127(Pt 18):4037-51. doi: 10.1242/jcs.151944. Epub 2014 Jul 29. J Cell Sci. 2014. PMID: 25074806 Free PMC article.
-
δ-Catenin Regulates Spine Architecture via Cadherin and PDZ-dependent Interactions.J Biol Chem. 2015 Apr 24;290(17):10947-57. doi: 10.1074/jbc.M114.632679. Epub 2015 Feb 27. J Biol Chem. 2015. PMID: 25724647 Free PMC article.
-
Cri-Du-Chat Syndrome: Clinical Profile and Chromosomal Microarray Analysis in Six Patients.Biomed Res Int. 2016;2016:5467083. doi: 10.1155/2016/5467083. Epub 2016 Apr 7. Biomed Res Int. 2016. PMID: 27144168 Free PMC article.
-
Culturing pyramidal neurons from the early postnatal mouse hippocampus and cortex.Nat Protoc. 2012 Sep;7(9):1741-54. doi: 10.1038/nprot.2012.099. Epub 2012 Aug 30. Nat Protoc. 2012. PMID: 22936216
References
-
- Abe K, Chisaka O, Van Roy F, Takeichi M. Stability of dendritic spines and synaptic contacts is controlled by alpha N-catenin. Nat Neurosci. 2004;7:357–363. - PubMed
-
- Abu-Elneel K, Ochiishi T, Medina M, Remedi M, Gastaldi L, Caceres A, Kosik KS. A delta-catenin signaling pathway leading to dendritic protrusions. J Biol Chem. 2008;283:32781–32791. - PubMed
-
- Alvarez VA, Sabatini BL. Anatomical and physiological plasticity of dendritic spines. Annu Rev Neurosci. 2007;30:79–97. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
