Drosophila Miro is required for both anterograde and retrograde axonal mitochondrial transport
- PMID: 19403812
- PMCID: PMC2693725
- DOI: 10.1523/JNEUROSCI.5417-08.2009
Drosophila Miro is required for both anterograde and retrograde axonal mitochondrial transport
Abstract
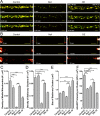
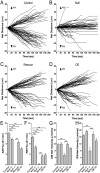
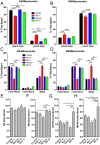
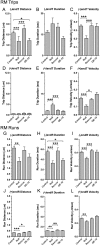
Microtubule-based transport of mitochondria into dendrites and axons is vital for sustaining neuronal function. Transport along microtubule tracks proceeds in a series of plus and minus end-directed movements that are facilitated by kinesin and dynein motors. How the opposing movements are controlled to achieve effective transport over large distances remains unclear. Previous studies showed that the conserved mitochondrial GTPase Miro is required for mitochondrial transport into axons and dendrites and serves as a Ca(2+) sensor that controls mitochondrial mobility. To directly examine Miro's significance for kinesin- and/or dynein-mediated mitochondrial motility, we live-imaged movements of GFP-tagged mitochondria in larval Drosophila motor axons upon genetic manipulations of Miro. Loss of Drosophila Miro (dMiro) reduced the effectiveness of both anterograde and retrograde mitochondrial transport by selectively impairing kinesin- or dynein-mediated movements, depending on the direction of net transport. Net anterogradely transported mitochondria exhibited reduced kinesin- but normal dynein-mediated movements. Net retrogradely transported mitochondria exhibited much shorter dynein-mediated movements, whereas kinesin-mediated movements were minimally affected. In both cases, the duration of short stationary phases increased proportionally. Overexpression (OE) of dMiro also impaired the effectiveness of mitochondrial transport. Finally, loss and OE of dMiro altered the length of mitochondria in axons through a mechanistically separate pathway. We suggest that dMiro promotes effective antero- and retrograde mitochondrial transport by extending the processivity of kinesin and dynein motors according to a mitochondrion's programmed direction of transport.
Figures



Similar articles
-
Miro's N-terminal GTPase domain is required for transport of mitochondria into axons and dendrites.J Neurosci. 2015 Apr 8;35(14):5754-71. doi: 10.1523/JNEUROSCI.1035-14.2015. J Neurosci. 2015. PMID: 25855186 Free PMC article.
-
A new mode of mitochondrial transport and polarized sorting regulated by Dynein, Milton and Miro.Development. 2016 Nov 15;143(22):4203-4213. doi: 10.1242/dev.138289. Epub 2016 Oct 5. Development. 2016. PMID: 27707795
-
The GTPase dMiro is required for axonal transport of mitochondria to Drosophila synapses.Neuron. 2005 Aug 4;47(3):379-93. doi: 10.1016/j.neuron.2005.06.027. Neuron. 2005. PMID: 16055062
-
The axonal transport of mitochondria.J Cell Sci. 2005 Dec 1;118(Pt 23):5411-9. doi: 10.1242/jcs.02745. J Cell Sci. 2005. PMID: 16306220 Free PMC article. Review.
-
Mitochondrial transport dynamics in axons and dendrites.Results Probl Cell Differ. 2009;48:107-39. doi: 10.1007/400_2009_20. Results Probl Cell Differ. 2009. PMID: 19582407 Review.
Cited by
-
An ER phospholipid hydrolase drives ER-associated mitochondrial constriction for fission and fusion.Elife. 2022 Nov 30;11:e84279. doi: 10.7554/eLife.84279. Elife. 2022. PMID: 36448541 Free PMC article.
-
Orchestrating mitochondria in neurons: Cytoskeleton as the conductor.Cytoskeleton (Hoboken). 2020 Mar;77(3-4):65-75. doi: 10.1002/cm.21585. Epub 2019 Dec 9. Cytoskeleton (Hoboken). 2020. PMID: 31782907 Free PMC article. Review.
-
Spatial control of neuronal metabolism through glucose-mediated mitochondrial transport regulation.Elife. 2018 Dec 18;7:e40986. doi: 10.7554/eLife.40986. Elife. 2018. PMID: 30561333 Free PMC article.
-
HUMMR, a hypoxia- and HIF-1alpha-inducible protein, alters mitochondrial distribution and transport.J Cell Biol. 2009 Jun 15;185(6):1065-81. doi: 10.1083/jcb.200811033. J Cell Biol. 2009. PMID: 19528298 Free PMC article.
-
Regulation of autophagy by protein post-translational modification.Lab Invest. 2015 Jan;95(1):14-25. doi: 10.1038/labinvest.2014.131. Epub 2014 Nov 3. Lab Invest. 2015. PMID: 25365205 Free PMC article. Review.
References
-
- Aberle H, Haghighi AP, Fetter RD, McCabe BD, Magalhães TR, Goodman CS. wishful thinking encodes a BMP type II receptor that regulates synaptic growth in Drosophila. Neuron. 2002;33:545–558. - PubMed
-
- Abramoff MD, Magelhaes PJ, Ram SJ. Image processing with ImageJ. Biophot Internat. 2004;11:36–42.
-
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. - PubMed
-
- Chada SR, Hollenbeck PJ. Mitochondrial movement and positioning in axons: the role of growth factor signaling. J Exp Biol. 2003;206:1985–1992. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
