Stimulus-specific adaptation in the inferior colliculus of the anesthetized rat
- PMID: 19403816
- PMCID: PMC2715893
- DOI: 10.1523/JNEUROSCI.4153-08.2009
Stimulus-specific adaptation in the inferior colliculus of the anesthetized rat
Abstract
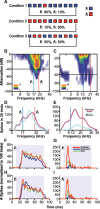
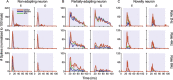
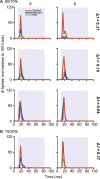
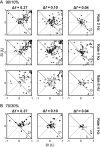
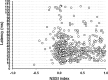
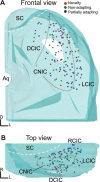
To identify sounds as novel, there must be some neural representation of commonly occurring sounds. Stimulus-specific adaptation (SSA) is a reduction in neural response to a repeated sound. Previous studies using an oddball stimulus paradigm have shown that SSA occurs at the cortex, but this study demonstrates that neurons in the inferior colliculus (IC) also show strong SSA using this paradigm. The majority (66%) of IC neurons showed some degree of SSA. Approximately 18% of neurons showed near-complete SSA. Neurons with SSA were found throughout the IC. Responses of IC neurons were reduced mainly during the onset component of the response, and latency was shorter in response to the oddball stimulus than to the standard. Neurons with near-complete SSA were broadly tuned to frequency, suggesting a high degree of convergence. Thus, some of the mechanisms that may underlie novelty detection and behavioral habituation to common sounds are already well developed at the midbrain.
Figures




References
-
- Bajo VM, Antunes FM, Covey E, Malmierca MS. Is there stimulus-specific adaptation in the auditory thalamus? In: Lopez-Poveda EA, Palmer AR, Meddis R, editors. Advances in auditory physiology, psychophysics and models. New York: Springer; 2009. in press.
-
- Bajo VM, Moore DR. Descending projections from the auditory cortex to the inferior colliculus in the gerbil, Meriones unguiculatus. J Comp Neurol. 2005;486:101–116. - PubMed
-
- Chechik G, Anderson MJ, Bar-Yosef O, Young ED, Tishby N, Nelken I. Reduction of information redundancy in the ascending auditory pathway. Neuron. 2006;51:359–368. - PubMed
-
- Condon CD, Weinberger NM. Habituation produces frequency-specific plasticity of receptive fields in the auditory cortex. Behav Neurosci. 1991;105:416–430. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
