Nicotinamide mononucleotide adenylyl transferase-mediated axonal protection requires enzymatic activity but not increased levels of neuronal nicotinamide adenine dinucleotide
- PMID: 19403820
- PMCID: PMC3162248
- DOI: 10.1523/JNEUROSCI.5469-08.2009
Nicotinamide mononucleotide adenylyl transferase-mediated axonal protection requires enzymatic activity but not increased levels of neuronal nicotinamide adenine dinucleotide
Abstract
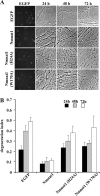
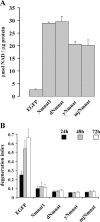
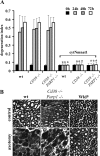
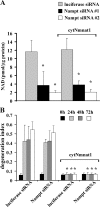
Axonal degeneration is a hallmark of many neurological disorders. Studies in animal models of neurodegenerative diseases indicate that axonal degeneration is an early event in the disease process, and delaying this process can lead to decreased progression of the disease and survival extension. Overexpression of the Wallerian degeneration slow (Wld(s)) protein can delay axonal degeneration initiated via axotomy, chemotherapeutic agents, or genetic mutations. The Wld(s) protein consists of the N-terminal portion of the ubiquitination factor Ube4b fused to the nicotinamide adenine dinucleotide (NAD(+)) biosynthetic enzyme nicotinamide mononucleotide adenylyl transferase 1 (Nmnat1). We previously showed that the Nmnat1 portion of this fusion protein was the critical moiety for Wld(s)-mediated axonal protection. Here, we describe the development of an automated quantitative assay for assessing axonal degeneration. This method successfully showed that Nmnat1 enzymatic activity is important for axonal protection as mutants with reduced enzymatic activity lacked axon protective activity. We also found that Nmnat enzymes with diverse sequences and structures from various species, including Drosophila melanogaster, Saccharomyces cerevisiae, and archaebacterium Methanocaldococcus jannaschii, which encodes a protein with no homology to eukaryotic Nmnat enzymes, all mediate robust axonal protection after axotomy. Besides the importance of Nmnat enzymatic activity, we did not observe changes in the steady-state NAD(+) level, and we found that inhibition of nicotinamide phosphoribosyltransferase (Nampt), which synthesizes substrate for Nmnat in mammalian cells, did not affect the protective activity of Nmnat1. These results provide the possibility of a role for new Nmnat enzymatic activity in axonal protection in addition to NAD(+) synthesis.
Figures


Similar articles
-
Transgenic mice expressing the Nmnat1 protein manifest robust delay in axonal degeneration in vivo.J Neurosci. 2009 May 20;29(20):6526-34. doi: 10.1523/JNEUROSCI.1429-09.2009. J Neurosci. 2009. PMID: 19458223 Free PMC article.
-
Nicotinamide mononucleotide adenylyltransferase expression in mitochondrial matrix delays Wallerian degeneration.J Neurosci. 2009 May 13;29(19):6276-84. doi: 10.1523/JNEUROSCI.4304-08.2009. J Neurosci. 2009. PMID: 19439605 Free PMC article.
-
Stimulation of nicotinamide adenine dinucleotide biosynthetic pathways delays axonal degeneration after axotomy.J Neurosci. 2006 Aug 16;26(33):8484-91. doi: 10.1523/JNEUROSCI.2320-06.2006. J Neurosci. 2006. PMID: 16914673 Free PMC article.
-
Why is NMNAT Protective against Neuronal Cell Death and Axon Degeneration, but Inhibitory of Axon Regeneration?Cells. 2019 Mar 21;8(3):267. doi: 10.3390/cells8030267. Cells. 2019. PMID: 30901919 Free PMC article. Review.
-
Axon degeneration: Mechanisms and implications of a distinct program from cell death.Neurochem Int. 2010 Mar;56(4):529-34. doi: 10.1016/j.neuint.2010.01.013. Epub 2010 Feb 1. Neurochem Int. 2010. PMID: 20117162 Review.
Cited by
-
SARM1 activation triggers axon degeneration locally via NAD⁺ destruction.Science. 2015 Apr 24;348(6233):453-7. doi: 10.1126/science.1258366. Epub 2015 Apr 23. Science. 2015. PMID: 25908823 Free PMC article.
-
SARM1-specific motifs in the TIR domain enable NAD+ loss and regulate injury-induced SARM1 activation.Proc Natl Acad Sci U S A. 2016 Oct 11;113(41):E6271-E6280. doi: 10.1073/pnas.1601506113. Epub 2016 Sep 26. Proc Natl Acad Sci U S A. 2016. PMID: 27671644 Free PMC article.
-
Isoform-specific targeting and interaction domains in human nicotinamide mononucleotide adenylyltransferases.J Biol Chem. 2010 Jun 11;285(24):18868-76. doi: 10.1074/jbc.M110.107631. Epub 2010 Apr 13. J Biol Chem. 2010. PMID: 20388704 Free PMC article.
-
Phosphatidylserine is a marker for axonal debris engulfment but its exposure can be decoupled from degeneration.Cell Death Dis. 2018 Nov 2;9(11):1116. doi: 10.1038/s41419-018-1155-z. Cell Death Dis. 2018. PMID: 30389906 Free PMC article.
-
NAD+ and sirtuins in aging and disease.Trends Cell Biol. 2014 Aug;24(8):464-71. doi: 10.1016/j.tcb.2014.04.002. Epub 2014 Apr 29. Trends Cell Biol. 2014. PMID: 24786309 Free PMC article. Review.
References
-
- An S, Kumar R, Sheets ED, Benkovic SJ. Reversible compartmentalization of de novo purine biosynthetic complexes in living cells. Science. 2008;320:103–106. - PubMed
-
- Araki T, Sasaki Y, Milbrandt J. Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science. 2004;305:1010–1013. - PubMed
-
- Berger F, Lau C, Dahlmann M, Ziegler M. Subcellular compartmentation and differential catalytic properties of the three human nicotinamide mononucleotide adenylyltransferase isoforms. J Biol Chem. 2005;280:36334–36341. - PubMed
-
- Bieganowski P, Brenner C. Discoveries of nicotinamide riboside as a nutrient and conserved NRK genes establish a Preiss-Handler independent route to NAD+ in fungi and humans. Cell. 2004;117:495–502. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
