The translational repressors Nanos and Pumilio have divergent effects on presynaptic terminal growth and postsynaptic glutamate receptor subunit composition
- PMID: 19403823
- PMCID: PMC2750846
- DOI: 10.1523/JNEUROSCI.0520-09.2009
The translational repressors Nanos and Pumilio have divergent effects on presynaptic terminal growth and postsynaptic glutamate receptor subunit composition
Abstract
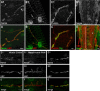
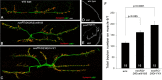
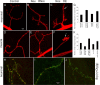
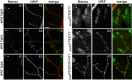
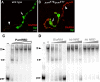
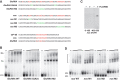
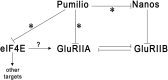
Pumilio (Pum) is a translational repressor that binds selectively to target mRNAs and recruits Nanos (Nos) as a corepressor. In the larval neuromuscular system, Pum represses expression of the translation factor eIF-4E and the glutamate receptor subunit GluRIIA. Here, we show that Nos, like Pum, is expressed at the neuromuscular junction (NMJ) and in neuronal cell bodies. Surprisingly, however, Nos and Pum have divergent functions on both the presynaptic and postsynaptic sides of the NMJ. In nos mutant and nos RNA interference larvae, the number of NMJ boutons is increased, whereas loss of Pum reduces the bouton number. On the postsynaptic side, Nos acts in opposition to Pum in regulating the subunit composition of the glutamate receptor. NMJ active zones are associated with GluRIIA- and GluRIIB-containing receptor clusters. Loss of Nos causes downregulation of GluRIIA and increases the levels of GluRIIB. Consistent with this finding, the electrophysiological properties of NMJs lacking postsynaptic Nos suggest that they use primarily GluRIIB-containing receptors. Nos can regulate GluRIIB in the absence of GluRIIA, suggesting that the effects of Nos on GluRIIB levels are at least partially independent of synaptic competition between GluRIIA and GluRIIB. Nos is a target for Pum repression, and Pum binds selectively to the 3' untranslated regions of the nos and GluRIIA mRNAs. Our results suggest a model in which regulatory interplay among Pum, Nos, GluRIIA, and GluRIIB could cause a small change in Pum activity to be amplified into a large shift in the balance between GluRIIA and GluRIIB synapses.
Figures



Similar articles
-
The translational repressor Pumilio regulates presynaptic morphology and controls postsynaptic accumulation of translation factor eIF-4E.Neuron. 2004 Nov 18;44(4):663-76. doi: 10.1016/j.neuron.2004.10.028. Neuron. 2004. PMID: 15541314
-
Discs-large (DLG) is clustered by presynaptic innervation and regulates postsynaptic glutamate receptor subunit composition in Drosophila.BMC Biol. 2005 Jan 8;3:1. doi: 10.1186/1741-7007-3-1. BMC Biol. 2005. PMID: 15638945 Free PMC article.
-
Dbo/Henji Modulates Synaptic dPAK to Gate Glutamate Receptor Abundance and Postsynaptic Response.PLoS Genet. 2016 Oct 13;12(10):e1006362. doi: 10.1371/journal.pgen.1006362. eCollection 2016 Oct. PLoS Genet. 2016. PMID: 27736876 Free PMC article.
-
Staufen targets coracle mRNA to Drosophila neuromuscular junctions and regulates GluRIIA synaptic accumulation and bouton number.Dev Biol. 2014 Aug 15;392(2):153-67. doi: 10.1016/j.ydbio.2014.06.007. Epub 2014 Jun 19. Dev Biol. 2014. PMID: 24951879 Free PMC article.
-
Receptor clustering: nothing succeeds like success.Curr Biol. 2004 Jun 8;14(11):R413-5. doi: 10.1016/j.cub.2004.05.031. Curr Biol. 2004. PMID: 15182686 Review.
Cited by
-
Presynaptic translation: stepping out of the postsynaptic shadow.Front Neural Circuits. 2009 Nov 4;3:17. doi: 10.3389/neuro.04.017.2009. eCollection 2009. Front Neural Circuits. 2009. PMID: 19915727 Free PMC article.
-
S6 kinase localizes to the presynaptic active zone and functions with PDK1 to control synapse development.J Cell Biol. 2011 Sep 19;194(6):921-35. doi: 10.1083/jcb.201101042. J Cell Biol. 2011. PMID: 21930778 Free PMC article.
-
Regulation of membrane excitability: a convergence on voltage-gated sodium conductance.Mol Neurobiol. 2015 Feb;51(1):57-67. doi: 10.1007/s12035-014-8674-0. Epub 2014 Mar 29. Mol Neurobiol. 2015. PMID: 24677068 Free PMC article. Review.
-
Nanos-mediated repression of hid protects larval sensory neurons after a global switch in sensitivity to apoptotic signals.Development. 2016 Jun 15;143(12):2147-59. doi: 10.1242/dev.132415. Epub 2016 May 4. Development. 2016. PMID: 27256879 Free PMC article.
-
dTip60 HAT activity controls synaptic bouton expansion at the Drosophila neuromuscular junction.PLoS One. 2011;6(10):e26202. doi: 10.1371/journal.pone.0026202. Epub 2011 Oct 27. PLoS One. 2011. PMID: 22046262 Free PMC article.
References
-
- Bergsten SE, Huang T, Chatterjee S, Gavis ER. Recognition and long-range interactions of a minimal nanos RNA localization signal element. Development. 2001;128:427–435. - PubMed
-
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
