How multiple conductances determine electrophysiological properties in a multicompartment model
- PMID: 19403824
- PMCID: PMC2821064
- DOI: 10.1523/JNEUROSCI.4438-08.2009
How multiple conductances determine electrophysiological properties in a multicompartment model
Abstract
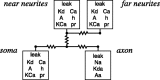
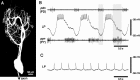
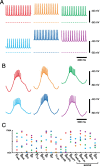
Most neurons have large numbers of voltage- and time-dependent currents that contribute to their electrical firing patterns. Because these currents are nonlinear, it can be difficult to determine the role each current plays in determining how a neuron fires. The lateral pyloric (LP) neuron of the stomatogastric ganglion of decapod crustaceans has been studied extensively biophysically. We constructed approximately 600,000 versions of a four-compartment model of the LP neuron and distributed 11 different currents into the compartments. From these, we selected approximately 1300 models that match well the electrophysiological properties of the biological neuron. Interestingly, correlations that were seen in the expression of channel mRNA in biological studies were not found across the approximately 1300 admissible LP neuron models, suggesting that the electrical phenotype does not require these correlations. We used cubic fits of the function from maximal conductances to a series of electrophysiological properties to ask which conductances predominantly influence input conductance, resting membrane potential, resting spike rate, phasing of activity in response to rhythmic inhibition, and several other properties. In all cases, multiple conductances contribute to the measured property, and the combinations of currents that strongly influence each property differ. These methods can be used to understand how multiple currents in any candidate neuron interact to determine the cell's electrophysiological behavior.
Figures





References
-
- Abbott LF. A network of oscillators. J Physics [A] 1990;23:3835–3859.
-
- Baro DJ, Harris-Warrick RM. Differential expression and targeting of K+ channel genes in the lobster pyloric central pattern generator. Ann N Y Acad Sci. 1998;860:281–295. - PubMed
-
- Brivanlou AH, Darnell JE., Jr Signal transduction and the control of gene expression. Science. 2002;295:813–818. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
