In search of adrenocortical stem and progenitor cells
- PMID: 19403887
- PMCID: PMC2726842
- DOI: 10.1210/er.2008-0039
In search of adrenocortical stem and progenitor cells
Abstract
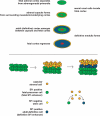
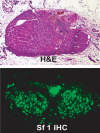
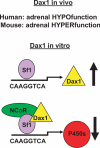
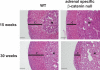
Scientists have long hypothesized the existence of tissue-specific (somatic) stem cells and have searched for their location in different organs. The theory that adrenocortical organ homeostasis is maintained by undifferentiated stem or progenitor cells can be traced back nearly a century. Similar to other organ systems, it is widely believed that these rare cells of the adrenal cortex remain relatively undifferentiated and quiescent until needed to replenish the organ, at which time they undergo proliferation and terminal differentiation. Historical studies examining cell cycle activation by label retention assays and regenerative potential by organ transplantation experiments suggested that the adrenocortical progenitors reside in the outer periphery of the adrenal gland. Over the past decade, the Hammer laboratory, building on this hypothesis and these observations, has endeavored to understand the mechanisms of adrenocortical development and organ maintenance. In this review, we summarize the current knowledge of adrenal organogenesis. We present evidence for the existence and location of adrenocortical stem/progenitor cells and their potential contribution to adrenocortical carcinomas. Data described herein come primarily from studies conducted in the Hammer laboratory with incorporation of important related studies from other investigators. Together, the work provides a framework for the emerging somatic stem cell field as it relates to the adrenal gland.
Figures



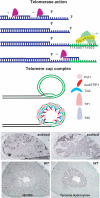


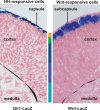
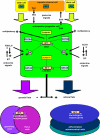
References
-
- Gottschau M 1883 Structur und embryonale Entwickelung der Nebennieren bei Saugethieren. Archiv fur Anat und Phys S:412–458
-
- Arnold J 1866 Ein Beitrag zur feineren Struktur und dem Chemismus der Nebennieren. Virchow Arch Patholog Anatomie Physiol Klin Med 35: 64–107
-
- Evelyn H-M 1927 A transitory zone in the adrenal cortex which shows age and sex relationships. Am J Anat 40:251–293
-
- Keegan CE, Hammer GD 2002 Recent insights into organogenesis of the adrenal cortex. Trends Endocrinol Metab 13:200–208 - PubMed
-
- Else T, Hammer GD 2005 Genetic analysis of adrenal absence: agenesis and aplasia. Trends Endocrinol Metab 16:458–468 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

