Constitutive activation of Beta-catenin in uterine stroma and smooth muscle leads to the development of mesenchymal tumors in mice
- PMID: 19403928
- PMCID: PMC2731978
- DOI: 10.1095/biolreprod.108.075648
Constitutive activation of Beta-catenin in uterine stroma and smooth muscle leads to the development of mesenchymal tumors in mice
Abstract
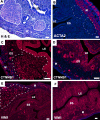
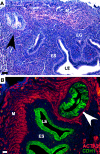
Leiomyomas and other mesenchymally derived tumors are the most common neoplasms of the female reproductive tract. Presently, very little is known about the etiology and progression of these tumors, which are the primary indication for hysterectomies. Dysregulated WNT signaling through beta-catenin is a well-established mechanism for tumorigenesis. We have developed a mouse model that expresses constitutively activated beta-catenin in uterine mesenchyme driven by the expression of Cre recombinase knocked into the Müllerian-inhibiting substance type II receptor promoter locus to investigate its effects on uterine endometrial stroma and myometrium. These mice show myometrial hyperplasia and develop mesenchymal tumors with 100% penetrance that exhibit histological and molecular characteristics of human leiomyomas and endometrial stromal sarcomas. By immunohistochemistry, we also show that both transforming growth factor beta and the mammalian target of rapamycin are induced by constitutive activation of beta-catenin. The prevalence of the tumors was greater in multiparous mice, suggesting that their development may be a hormonally driven process or that changes in uterine morphology during pregnancy and after parturition induce injury and repair mechanisms that stimulate tumorigenesis from stem/progenitor cells, which normally do not express constitutively activated beta-catenin. Additionally, adenomyosis and endometrial gland hyperplasia were occasionally observed in some mice. These results show evidence suggesting that dysregulated, stromal, and myometrial WNT/beta-catenin signaling has pleiotropic effects on uterine function and tumorigenesis.
Figures




Similar articles
-
Paracrine activation of WNT/β-catenin pathway in uterine leiomyoma stem cells promotes tumor growth.Proc Natl Acad Sci U S A. 2013 Oct 15;110(42):17053-8. doi: 10.1073/pnas.1313650110. Epub 2013 Sep 30. Proc Natl Acad Sci U S A. 2013. PMID: 24082114 Free PMC article. Clinical Trial.
-
Impaired down-regulation of E-cadherin and beta-catenin protein expression in endometrial epithelial cells in the mid-secretory endometrium of infertile patients with endometriosis.J Clin Endocrinol Metab. 2010 Jul;95(7):3437-45. doi: 10.1210/jc.2009-2713. Epub 2010 Apr 21. J Clin Endocrinol Metab. 2010. PMID: 20410224
-
Epidemiological and genetic clues for molecular mechanisms involved in uterine leiomyoma development and growth.Hum Reprod Update. 2015 Sep-Oct;21(5):593-615. doi: 10.1093/humupd/dmv030. Epub 2015 Jul 3. Hum Reprod Update. 2015. PMID: 26141720 Free PMC article. Review.
-
MED12 alterations in both human benign and malignant uterine soft tissue tumors.PLoS One. 2012;7(6):e40015. doi: 10.1371/journal.pone.0040015. Epub 2012 Jun 29. PLoS One. 2012. PMID: 22768200 Free PMC article.
-
Wnt/β-catenin signaling pathway in uterine leiomyoma: role in tumor biology and targeting opportunities.Mol Cell Biochem. 2021 Sep;476(9):3513-3536. doi: 10.1007/s11010-021-04174-6. Epub 2021 May 17. Mol Cell Biochem. 2021. PMID: 33999334 Free PMC article. Review.
Cited by
-
Animal Models of Adenomyosis.Semin Reprod Med. 2020 May;38(2-03):168-178. doi: 10.1055/s-0040-1718741. Epub 2020 Oct 26. Semin Reprod Med. 2020. PMID: 33105508 Free PMC article. Review.
-
Upregulation of miR‑33b promotes endometriosis via inhibition of Wnt/β‑catenin signaling and ZEB1 expression.Mol Med Rep. 2019 Mar;19(3):2144-2152. doi: 10.3892/mmr.2019.9870. Epub 2019 Jan 17. Mol Med Rep. 2019. PMID: 30664209 Free PMC article.
-
Role of Transforming Growth Factor β in Uterine Fibroid Biology.Int J Mol Sci. 2017 Nov 17;18(11):2435. doi: 10.3390/ijms18112435. Int J Mol Sci. 2017. PMID: 29149020 Free PMC article. Review.
-
Uterine gland formation in mice is a continuous process, requiring the ovary after puberty, but not after parturition.Biol Reprod. 2011 Nov;85(5):954-64. doi: 10.1095/biolreprod.111.091470. Epub 2011 Jul 6. Biol Reprod. 2011. PMID: 21734259 Free PMC article.
-
Deletion of tuberous sclerosis 1 in somatic cells of the murine reproductive tract causes female infertility.Endocrinology. 2012 Jan;153(1):404-16. doi: 10.1210/en.2011-1191. Epub 2011 Nov 29. Endocrinology. 2012. PMID: 22128018 Free PMC article.
References
-
- Cook JD, Walker CL.Treatment strategies for uterine leiomyoma: the role of hormonal modulation. Semin Reprod Med 2004; 22: 105–111.. - PubMed
-
- Walker CL, Stewart EA.Uterine fibroids: the elephant in the room. Science 2005; 308: 1589–1592.. - PubMed
-
- Mikels AJ, Nusse R.Wnts as ligands: processing, secretion and reception. Oncogene 2006; 25: 7461–7468.. - PubMed
-
- Polakis P.Wnt signaling and cancer. Genes Dev 2000; 14: 1837–1851.. - PubMed
-
- Ng TL, Gown AM, Barry TS, Cheang MC, Chan AK, Turbin DA, Hsu FD, West RB, Nielsen TO.Nuclear beta-catenin in mesenchymal tumors. Mod Pathol 2005; 18: 68–74.. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

