Therapies for hyperglycaemia-induced diabetic complications: from animal models to clinical trials
- PMID: 19404313
- PMCID: PMC7097138
- DOI: 10.1038/nrd2476
Therapies for hyperglycaemia-induced diabetic complications: from animal models to clinical trials
Abstract
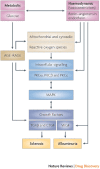
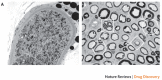
Long-term diabetes increases the likelihood of developing secondary damage to numerous systems, and these complications represent a substantial cause of morbidity and mortality. Establishing the causes of diabetes remains the key step towards eradicating the disease, but the prevention and amelioration of diabetic complications is equally important for the millions of individuals who already have the disease or are likely to develop it before prophylaxis or a cure become routinely available. In this Review, we focus on four common complications of diabetes, discuss the range of pathologies that are precipitated by hyperglycaemia and highlight emerging targets for therapeutic intervention.
Conflict of interest statement
M.E.C. has received research grants from Synvista, Speedel and Astra Zeneca, which are the manufacturers of algebrium, avosentan and candesartan, respectively. He has also received honoraria from GlaxoSmithKline, Servier and Astra Zeneca, which are the manufacturers of rosiglitazone, perindopril and candesartan, respectively.
Figures
References
-
- Botero D, Wolfsdorf JI. Diabetes mellitus in children and adolescents. Arch. Med. Res. 2005;36:281–290. - PubMed
-
- Turner RC, Holman RR. Lessons from UK prospective diabetes study. Diabetes Res. Clin. Pract. 1995;28:S151–S157. - PubMed
-
- Gabbay KH. The sorbitol pathway and the complications of diabetes. N. Engl. J. Med. 1973;288:831–836. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

