Local control of excitation-contraction coupling in human embryonic stem cell-derived cardiomyocytes
- PMID: 19404384
- PMCID: PMC2671137
- DOI: 10.1371/journal.pone.0005407
Local control of excitation-contraction coupling in human embryonic stem cell-derived cardiomyocytes
Abstract
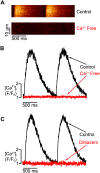
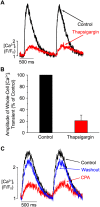
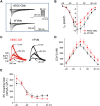
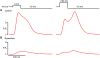
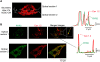
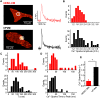
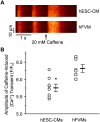
We investigated the mechanisms of excitation-contraction (EC) coupling in human embryonic stem cell-derived cardiomyocytes (hESC-CMs) and fetal ventricular myocytes (hFVMs) using patch-clamp electrophysiology and confocal microscopy. We tested the hypothesis that Ca(2+) influx via voltage-gated L-type Ca(2+) channels activates Ca(2+) release from the sarcoplasmic reticulum (SR) via a local control mechanism in hESC-CMs and hFVMs. Field-stimulated, whole-cell [Ca(2+)](i) transients in hESC-CMs required Ca(2+) entry through L-type Ca(2+) channels, as evidenced by the elimination of such transients by either removal of extracellular Ca(2+) or treatment with diltiazem, an L-type channel inhibitor. Ca(2+) release from the SR also contributes to the [Ca(2+)](i) transient in these cells, as evidenced by studies with drugs interfering with either SR Ca(2+) release (i.e. ryanodine and caffeine) or reuptake (i.e. thapsigargin and cyclopiazonic acid). As in adult ventricular myocytes, membrane depolarization evoked large L-type Ca(2+) currents (I(Ca)) and corresponding whole-cell [Ca(2+)](i) transients in hESC-CMs and hFVMs, and the amplitude of both I(Ca) and the [Ca(2+)](i) transients were finely graded by the magnitude of the depolarization. hESC-CMs exhibit a decreasing EC coupling gain with depolarization to more positive test potentials, "tail" [Ca(2+)](i) transients upon repolarization from extremely positive test potentials, and co-localized ryanodine and sarcolemmal L-type Ca(2+) channels, all findings that are consistent with the local control hypothesis. Finally, we recorded Ca(2+) sparks in hESC-CMs and hFVMs. Collectively, these data support a model in which tight, local control of SR Ca(2+) release by the I(Ca) during EC coupling develops early in human cardiomyocytes.
Conflict of interest statement
Figures
References
-
- Bers DM. Excitation-contraction coupling and cardiac contractile force. Dordrecht, The Netherlands: 2001.
-
- Cheng H, Lederer WJ. Calcium sparks. Physiol Rev. 2008;88:1491–1545. - PubMed
-
- Cannell MB, Cheng H, Lederer WJ. The control of calcium release in heart muscle. Science. 1995;268:1045–1049. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

