Dissociable effects of noradrenaline, dopamine, and serotonin uptake blockade on stop task performance in rats
- PMID: 19404616
- PMCID: PMC2705723
- DOI: 10.1007/s00213-009-1537-0
Dissociable effects of noradrenaline, dopamine, and serotonin uptake blockade on stop task performance in rats
Abstract
Rationale: The stop-signal paradigm measures the ability to stop a motor response after its execution has been initiated. Impairments in inhibiting inappropriate behavior and prolonged stop-signal reaction times (SSRTs) are characteristic of several psychiatric disorders, most notably attention deficit/hyperactivity disorder. While there is relative consensus regarding the anatomical substrates of behavioral inhibition, the neurochemical imbalance responsible for the deficits in stopping displayed by impulsive individuals is still a matter of debate.
Objective: The aim of this study was to investigate the effects of manipulating brain monoamine levels on stop task parameters.
Methods: Lister-hooded rats were trained on the rodent version of the stop-signal task and administered different monoamine transporter inhibitors: citalopram, which selectively blocks the serotonin transporter; atomoxetine, which selectively blocks the noradrenaline transporter; and GBR-12909, which selectively blocks the dopamine transporter (DAT), and the alpha-2 adrenergic agonist guanfacine.
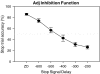
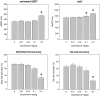
Results: Atomoxetine speeded SSRT and increased accuracy for go-trials. Citalopram slowed go reaction time and decreased go accuracy at the highest dose (1 mg/kg). GBR-12909 speeded go reaction time and impaired both go and stop accuracy. Guanfacine negatively modulated all principal stop and go measures at the highest dose used (0.3 mg/kg).
Conclusions: The results suggest that atomoxetine exerts its beneficial effects on SSRT via its action on noradrenaline re-uptake, as the specific DAT blocker GBR-12909 and serotonin reuptake blockade had only minor effects on SSRT. The speeding of the go reaction time by dopamine reuptake blockade is consistent with the hypothesis that the hypothetical stop and go processes are modulated by distinct monoaminergic systems.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

