Elution of gentamicin and vancomycin from polymethylmethacrylate beads and hip spacers in vivo
- PMID: 19404802
- PMCID: PMC2823164
- DOI: 10.3109/17453670902884700
Elution of gentamicin and vancomycin from polymethylmethacrylate beads and hip spacers in vivo
Abstract
Background and purpose: Late infections after total hip arthroplasty are still a problem. Treatment procedures include resection arthroplasty with implantation of antibiotic-loaded beads or implantation of an antibiotic-impreganted spacer. However, little is known about antibiotic elution from bone cement beyond the first 2-3 postoperative days in humans.
Methods: 17 hip spacers (80 g PMMA, 1g gentamicin, and 4 g vancomycin) and 11 chains (40 g PMMA, 0.5 g gentamicin, and 2 g vancomycin) in 28 patients were studied. The release of both agents was measured in the drainage fluid on a daily basis. The drains were left in situ until less than 50 mL was produced per day. The elution of both antibiotics was determined by fluorescence polarization immunoassay. Systemic antibiotics were given postoperatively according to antibiogram. If possible, no gentamicin or vancomycin was given.
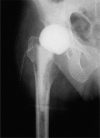
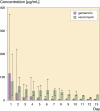
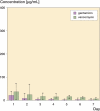
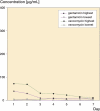
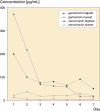
Results: Peak mean concentrations from beads and spacers were reached for gentamicin (1,160 (12-371) microg/mL and 21 (0.7-39) microg/mL, respectively) and for vancomycin (80 (21-198) microg/mL and 37 (3.3-72) microg/mL) on day 1. The last concentrations to be determined were 3.7 microg/mL gentamicin and 23 microg/mL vancomycin in the beads group after 13 days, and 1.9 microg/mL gentamicin and 6.6 microg/mL vancomycin in the spacer group after 7 days. Between the fifth and seventh day, an intermittent increase in elution of vancomycin from both beads and spacers and of gentamicin from spacers was noticed. No renal or hepatic dysfunction was observed.
Interpretation: Beads showed higher elution characteristics in vivo than the spacers due to their larger surface area; however, a great amount of inter-subject variability was seen for both beads and spacers. The inferior elution properties of spacers emphasize the importance of additional systemic antibiotics for this treatment procedure during the postoperative period. Future studies should clarify whether the dose of antibiotics or length of antibiotic therapy may be reduced in the case of bead implantation, without jeopardizing the control of infection.
Figures
References
-
- Adams K, Couch L, Cierny G, Calhoun J, Mader JT. In vitro an din vivo evaluation of antibiotic diffusion from antibiotic-impregnated polymethylmethacrylate beads. Clin Orthop. 1992;(278):244–52. - PubMed
-
- Anagnostakos K, Kelm J, Regitz T, Schmitt E, Jung W. In vitro evaluation of antibiotic release from and bacterai growth inhibition by antibiotic-loaded acrylic bone cement spacers. J Biomed Mater Res B Appl Biomater. 2005;72(2):373–8. - PubMed
-
- Anagnostakos K, Fürst O, Kelm J. Antibiotic-impregnated hip spacers: Current status. Acta Orthop. 2006;77(4):628–37. - PubMed
-
- Bertazzonni Minelli E, Benini A, Magnan B, Bartolozzi P. Release of gentamicin and vancomycin from temporary human hip spacers in two-stage revision of infected arthroplasty. J Antimicrob Chemother. 2004;53(2):329–34. - PubMed
-
- Buchholz HW, Engelbrecht H. Depot effects of various antibiotics mixed with Palacos resins. Chirurg. 1970;41(11):511–5. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
