Altered mineralization of human osteoarthritic osteoblasts is attributable to abnormal type I collagen production
- PMID: 19404930
- PMCID: PMC5250342
- DOI: 10.1002/art.24489
Altered mineralization of human osteoarthritic osteoblasts is attributable to abnormal type I collagen production
Abstract
Objective: Bone tissue in osteoarthritis (OA) is composed of abundant undermineralized osteoid matrix. The aim of this study was to investigate the mechanisms responsible for this abnormal matrix, using in vitro OA subchondral osteoblasts.
Methods: Primary normal and OA osteoblasts were prepared from tibial plateaus. Phenotype was determined by alkaline phosphatase activity, and osteocalcin, osteopontin, prostaglandin E2 (PGE2), and transforming growth factor beta1 (TGFbeta1) were assessed by enzyme-linked immunosorbent assay. Expression of COL1A1 and COL1A2 was determined by real-time polymerase chain reaction. The production of type I collagen was determined by the release of its C-terminal propeptide and Western blot analysis. In vitro mineralization was evaluated by alizarin red staining. Inhibition of TGFbeta1 expression was performed using a small interfering RNA technique.
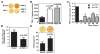
Results: Mineralization of OA osteoblasts was reduced compared with mineralization of normal osteoblasts, even in the presence of bone morphogenetic protein 2 (BMP-2). Alkaline phosphatase and osteocalcin levels were elevated in OA osteoblasts compared with normal osteoblasts, whereas osteopontin levels were similar. The COL1A1-to-COL1A2 messenger RNA ratio was 3-fold higher in OA osteoblasts compared with normal osteoblasts, and the production of collagen by OA osteoblasts was increased. Because TGFbeta1 inhibits BMP-2-dependent mineralization, and because TGFbeta1 levels are approximately 4-fold higher in OA osteoblasts than in normal osteoblasts, inhibiting TGFbeta1 levels in OA osteoblasts corrected the abnormal COL1A1-to-COL1A2 ratio and increased alizarin red staining.
Conclusion: Elevated TGFbeta1 levels in OA osteoblasts are responsible, in part, for the abnormal ratio of COL1A1 to COL1A2 and for the abnormal production of mature type I collagen. This abnormal COL1A1-to-COL1A2 ratio generates a matrix that blunts mineralization in OA osteoblasts.
Figures
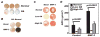



References
-
- Hilal G, Martel-Pelletier J, Pelletier JP, Ranger P, Lajeunesse D. Osteoblast-like cells from human subchondral osteoarthritic bone demonstrate an altered phenotype in vitro: possible role in subchondral bone sclerosis. Arthritis Rheum. 1998;41:891–9. - PubMed
-
- Westacott CI, Webb GR, Warnock MG, Sims JV, Elson CJ. Alteration of cartilage metabolism by cells from osteoarthritic bone. Arthritis Rheum. 1998;40:1282–91. - PubMed
-
- Radin EL, Rose RM. Role of subchondral bone in the initiation and progression of cartilage damage. Clin Orthop. 1986;213:34–40. - PubMed
-
- Li B, Aspden RM. Composition and mechanical properties of cancellous bone from the femoral head of patients with osteoporosis or osteoarthritis. J Bone Miner Res. 1997;12:641–51. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

