Targeting AKT with the proapoptotic peptide, TAT-CTMP: a novel strategy for the treatment of human pancreatic adenocarcinoma
- PMID: 19405118
- PMCID: PMC3645501
- DOI: 10.1002/ijc.24424
Targeting AKT with the proapoptotic peptide, TAT-CTMP: a novel strategy for the treatment of human pancreatic adenocarcinoma
Abstract
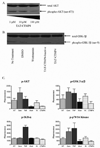
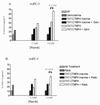
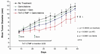
Pancreatic adenocarcinoma carries an ominous prognosis and has little effective treatment. Several studies have demonstrated that the potently antiapoptotic phosphatidyl inositol 3'-kinase (PI3K)-protein kinase B/AKT pathway is active in pancreas cancer. A recent study identified an endogenous AKT antagonist, carboxyl terminal modulator protein (CTMP). CTMP inhibits the phosphorylation of AKT, preventing full activation of the kinase. We screened several cell permeable peptides from the N-terminal domain of CTMP (termed TAT-CTMP1-4) in vitro and found one that caused significant apoptosis in pancreatic adenocarcinoma cell lines. An inactive variant of this peptide was synthesized and used as a negative control. In all cell lines tested, TAT-CTMP4 induced a dose-dependent increase in apoptosis as detected by %-TUNEL positive cells and %-active caspase-3 (% active caspase-3 ranged from 31.2 to 61.9 at the highest dose tested (10 microM). A screening of various cell and tissue types revealed that the proapoptotic activity was highest in pancreatic adenocarcinoma. TAT-CTMP induced similar levels of active caspase-3 as several other known inducers of apoptosis: gemcitabine, radiation therapy, wortmannin and recombinant tumor necrosis factor (TNF)-alpha. No apoptosis was observed in donor human peripheral blood mononuclear cells (PBMC, p < 0.01). We further showed that TAT-CTMP4 could augment either gemcitabine chemotherapy or radiation therapy, standard therapies for pancreas cancer. Pancreatic adenocarcinoma xenografts treated with a single dose of TAT-CTMP4 demonstrated a marked increase in caspase-3 positive tumor cells when compared with untreated controls. Additionally, pancreatic adenocarcinoma allografts treated with intratumoral TAT-CTMP and systemic gemcitabine displayed a significantly smaller tumor burden while undergoing treatment than mice in control groups (p < 0.001). These data indicate that inhibiting AKT with CTMP may be of therapeutic benefit in the treatment of pancreatic adenocarcinoma and, when combined with established therapies, may result in an increase in tumor cell death.
Conflict of interest statement
Figures




Similar articles
-
Inhibition of phosphatidylinositide 3-kinase enhances gemcitabine-induced apoptosis in human pancreatic cancer cells.Cancer Res. 2000 Oct 1;60(19):5451-5. Cancer Res. 2000. PMID: 11034087
-
Inhibition of AKT2 enhances sensitivity to gemcitabine via regulating PUMA and NF-κB signaling pathway in human pancreatic ductal adenocarcinoma.Int J Mol Sci. 2012;13(1):1186-1208. doi: 10.3390/ijms13011186. Epub 2012 Jan 20. Int J Mol Sci. 2012. PMID: 22312312 Free PMC article.
-
Silencing pancreatic adenocarcinoma upregulated factor (PAUF) increases the sensitivity of pancreatic cancer cells to gemcitabine.Tumour Biol. 2016 Jun;37(6):7555-64. doi: 10.1007/s13277-015-4641-2. Epub 2015 Dec 18. Tumour Biol. 2016. PMID: 26684804
-
HMGA1 is a molecular determinant of chemoresistance to gemcitabine in pancreatic adenocarcinoma.Clin Cancer Res. 2008 Mar 1;14(5):1470-7. doi: 10.1158/1078-0432.CCR-07-1450. Clin Cancer Res. 2008. PMID: 18316571 Free PMC article.
-
Effects of the epidermal growth factor receptor inhibitor OSI-774, Tarceva, on downstream signaling pathways and apoptosis in human pancreatic adenocarcinoma.Mol Cancer Ther. 2002 Aug;1(10):777-83. Mol Cancer Ther. 2002. PMID: 12492110
Cited by
-
Overview of carboxyl‑terminal modulator protein 1 and its importance in various metabolic regulations (Review).Mol Med Rep. 2024 Sep;30(3):158. doi: 10.3892/mmr.2024.13282. Epub 2024 Jul 12. Mol Med Rep. 2024. PMID: 38994770 Free PMC article. Review.
-
CAVPENET Peptide Inhibits Prostate Cancer Cells Proliferation and Migration through PP1γ-Dependent Inhibition of AKT Signaling.Pharmaceutics. 2024 Sep 12;16(9):1199. doi: 10.3390/pharmaceutics16091199. Pharmaceutics. 2024. PMID: 39339236 Free PMC article.
-
Irinotecan synergistically enhances the antiproliferative and proapoptotic effects of axitinib in vitro and improves its anticancer activity in vivo.Neoplasia. 2011 Mar;13(3):217-29. doi: 10.1593/neo.101334. Neoplasia. 2011. PMID: 21390185 Free PMC article.
-
ST6Gal-I sialyltransferase promotes chemoresistance in pancreatic ductal adenocarcinoma by abrogating gemcitabine-mediated DNA damage.J Biol Chem. 2018 Jan 19;293(3):984-994. doi: 10.1074/jbc.M117.808584. Epub 2017 Nov 30. J Biol Chem. 2018. PMID: 29191829 Free PMC article.
-
Amphiregulin regulates the activation of ERK and Akt through epidermal growth factor receptor and HER3 signals involved in the progression of pancreatic cancer.Cancer Sci. 2010 Nov;101(11):2351-60. doi: 10.1111/j.1349-7006.2010.01671.x. Cancer Sci. 2010. PMID: 20726858 Free PMC article.
References
-
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71–96. - PubMed
-
- Sener SF, Fremgen A, Menck HR, Winchester DP. Pancreatic cancer: report of treatment and survival trends for 100,313 patients diagnosed from 1985–1995, using the National Cancer Database. J Am Coll Surg. 1999;189:1–7. - PubMed
-
- Prochazkova J, Lichnovsky V, Kylarova D, Erdosova B, Vranka P. Involvement of p53 and Bcl-2 family proteins in regulating programmed cell death and proliferation in human embryogenesis. Gen Physiol Biophys. 2004;23(2):209–229. - PubMed
-
- Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219. - PubMed
-
- Debatin KM, Krammer PH. Death receptors in chemotherapy and cancer. Oncogene. 2004;23(16):2950–2966. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

