Unraveling a multifactorial late-onset disease: from genetic susceptibility to disease mechanisms for age-related macular degeneration
- PMID: 19405847
- PMCID: PMC3469316
- DOI: 10.1146/annurev.genom.9.081307.164350
Unraveling a multifactorial late-onset disease: from genetic susceptibility to disease mechanisms for age-related macular degeneration
Abstract
Aging-associated neurodegenerative diseases significantly influence the quality of life of affected individuals. Genetic approaches, combined with genomic technology, have provided powerful insights into common late-onset diseases, such as age-related macular degeneration (AMD). Here, we discuss current findings on the genetics of AMD to highlight areas of rapid progress and new challenges. We also attempt to integrate available genetic and biochemical data with cellular pathways involved in aging to formulate an integrated model of AMD pathogenesis.
Figures
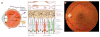
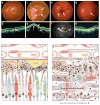

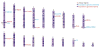



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

