Evidence synthesis as the key to more coherent and efficient research
- PMID: 19405972
- PMCID: PMC2681473
- DOI: 10.1186/1471-2288-9-29
Evidence synthesis as the key to more coherent and efficient research
Abstract
Background: Systematic review and meta-analysis currently underpin much of evidence-based medicine. Such methodologies bring order to previous research, but future research planning remains relatively incoherent and inefficient.
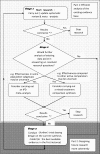
Methods: To outline a framework for evaluation of health interventions, aimed at increasing coherence and efficiency through i) making better use of information contained within the existing evidence-base when designing future studies; and ii) maximising the information available and thus potentially reducing the need for future studies.
Results: The framework presented insists that an up-to-date meta-analysis of existing randomised controlled trials (RCTs) should always be considered before future trials are conducted. Such a meta-analysis should inform critical design issues such as sample size determination. The contexts in which the use of individual patient data meta-analysis and mixed treatment comparisons modelling may be beneficial before further RCTs are conducted are considered. Consideration should also be given to how any newly planned RCTs would contribute to the totality of evidence through its incorporation into an updated meta-analysis. We illustrate how new RCTs can have very low power to change inferences of an existing meta-analysis, particularly when between study heterogeneity is taken into consideration.
Conclusion: While the collation of existing evidence as the basis for clinical practice is now routine, a more coherent and efficient approach to planning future RCTs to strengthen the evidence base needs to be developed. The framework presented is a proposal for how this situation can be improved.
Figures

References
-
- Higgins JPT, Green S, editors. The Cochrane Library. 3. Chichester, UK: John Wiley & Sons, Ltd; 2005. Cochrane Handbook for Systematic Reviews of Interventions 4.2.5.
-
- Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D. Improving the quality of reporting of meta-analysis of randomised controlled trials: the QUOROM statement. Onkologie. 2000;23(6):597–602. - PubMed
-
- Clarke L, Clarke M, Clarke T. How useful are Cochrane reviews in identifying research needs? Journal of Health Services Research & Policy. 2007;12:101–103. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

