Changing patterns of West Nile virus transmission: altered vector competence and host susceptibility
- PMID: 19406093
- PMCID: PMC2695027
- DOI: 10.1051/vetres/2009026
Changing patterns of West Nile virus transmission: altered vector competence and host susceptibility
Abstract
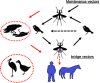
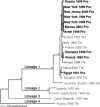
West Nile virus (WNV) is a flavivirus (Flaviviridae) transmitted between Culex spp. mosquitoes and avian hosts. The virus has dramatically expanded its geographic range in the past ten years. Increases in global commerce, climate change, ecological factors and the emergence of novel viral genotypes likely play significant roles in the emergence of this virus; however, the exact mechanism and relative importance of each is uncertain. Previously WNV was primarily associated with febrile illness of children in endemic areas, but it was identified as a cause of neurological disease in humans in 1994. This modulation in disease presentation could be the result of the emergence of a more virulent genotype as well as the progression of the virus into areas in which the age structure of immunologically naïve individuals makes them more susceptible to severe neurological disease. Since its introduction to North America in 1999, a novel WNV genotype has been identified that has been demonstrated to disseminate more rapidly and with greater efficiency at elevated temperatures than the originally introduced strain, indicating the potential importance of temperature as a selective criteria for the emergence of WNV genotypes with increased vectorial capacity. Even prior to the North American introduction, a mutation associated with increased replication in avian hosts, identified to be under adaptive evolutionary pressure, has been identified, indicating that adaptation for increased replication within vertebrate hosts could play a role in increased transmission efficiency. Although stable in its evolutionary structure, WNV has demonstrated the capacity for rapidly adapting to both vertebrate hosts and invertebrate vectors and will likely continue to exploit novel ecological niches as it adapts to novel transmission foci.
Figures

References
-
- Adams S.C., Broom A.K., Sammels L.M., Hartnett A.C., Howard M.J., Coelen R.J.. et al. Glycosylation and antigenic variation among Kunjin virus isolates. Virology. 1995;206:49–56. - PubMed
-
- Ahmed T., Hayes C.G., Baqar S.. Comparison of vector competence for West Nile virus of colonized populations of Culex tritaeniorhynchus from southern Asia and the Far East. Southeast Asian J. Trop. Med. Public Health. 1979;10:498–504. - PubMed
-
- Akhter R., Hayes C.G., Baqar S., Reisen W.K.. West Nile virus in Pakistan III. Comparative vector capability of Culex tritaeniorhynchus and eight other species of mosquitoes. Trans. R. Soc. Trop. Med. Hyg. 1982;76:449–453. - PubMed
-
- Anonymous. Outbreak of West Nile-like viral encephalitis – New York, 1999. MMWR Morb. Mortal. Wkly Rep. 1999;48:845–849. - PubMed
-
- Anonymous. Update: West Nile-like viral encephalitis – New York, 1999. MMWR Morb. Mortal. Wkly Rep. 1999;48:890–892. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
