Functions and evolution of selenoprotein methionine sulfoxide reductases
- PMID: 19406207
- PMCID: PMC3062201
- DOI: 10.1016/j.bbagen.2009.04.014
Functions and evolution of selenoprotein methionine sulfoxide reductases
Abstract
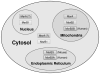
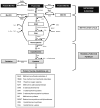
Methionine sulfoxide reductases (Msrs) are thiol-dependent enzymes which catalyze conversion of methionine sulfoxide to methionine. Three Msr families, MsrA, MsrB, and fRMsr, are known. MsrA and MsrB are responsible for the reduction of methionine-S-sulfoxide and methionine-R-sulfoxide residues in proteins, respectively, whereas fRMsr reduces free methionine-R-sulfoxide. Besides acting on proteins, MsrA can additionally reduce free methionine-S-sulfoxide. Some MsrAs and MsrBs evolved to utilize catalytic selenocysteine. This includes MsrB1, which is a major MsrB in cytosol and nucleus in mammalian cells. Specialized machinery is used for insertion of selenocysteine into MsrB1 and other selenoproteins at in-frame UGA codons. Selenocysteine offers catalytic advantage to the protein repair function of Msrs, but also makes these proteins dependent on the supply of selenium and requires adjustments in their strategies for regeneration of active enzymes. Msrs have roles in protecting cellular proteins from oxidative stress and through this function they may regulate lifespan in several model organisms.
Figures

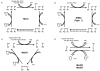

Similar articles
-
Methionine sulfoxide reductases: selenoprotein forms and roles in antioxidant protein repair in mammals.Biochem J. 2007 Nov 1;407(3):321-9. doi: 10.1042/BJ20070929. Biochem J. 2007. PMID: 17922679 Review.
-
The methionine sulfoxide reduction system: selenium utilization and methionine sulfoxide reductase enzymes and their functions.Antioxid Redox Signal. 2013 Sep 20;19(9):958-69. doi: 10.1089/ars.2012.5081. Epub 2013 Jan 22. Antioxid Redox Signal. 2013. PMID: 23198996 Free PMC article. Review.
-
MsrB1 (methionine-R-sulfoxide reductase 1) knock-out mice: roles of MsrB1 in redox regulation and identification of a novel selenoprotein form.J Biol Chem. 2009 Feb 27;284(9):5986-93. doi: 10.1074/jbc.M805770200. Epub 2008 Nov 6. J Biol Chem. 2009. PMID: 18990697 Free PMC article.
-
Regulation of selenoproteins and methionine sulfoxide reductases A and B1 by age, calorie restriction, and dietary selenium in mice.Antioxid Redox Signal. 2010 Apr 1;12(7):829-38. doi: 10.1089/ars.2009.2895. Antioxid Redox Signal. 2010. PMID: 19769460 Free PMC article.
-
The selenoprotein methionine sulfoxide reductase B1 (MSRB1).Free Radic Biol Med. 2022 Oct;191:228-240. doi: 10.1016/j.freeradbiomed.2022.08.043. Epub 2022 Sep 7. Free Radic Biol Med. 2022. PMID: 36084791 Review.
Cited by
-
[Effects of titanium dioxide nanoparticles on fecal metabolome in rats after oral administration for 90 days].Beijing Da Xue Xue Bao Yi Xue Ban. 2020 Jun 18;52(3):457-463. doi: 10.19723/j.issn.1671-167X.2020.03.010. Beijing Da Xue Xue Bao Yi Xue Ban. 2020. PMID: 32541978 Free PMC article. Chinese.
-
Lack of the antioxidant enzyme methionine sulfoxide reductase A in mice impairs RPE phagocytosis and causes photoreceptor cone dysfunction.Redox Biol. 2021 Jun;42:101918. doi: 10.1016/j.redox.2021.101918. Epub 2021 Feb 26. Redox Biol. 2021. PMID: 33674251 Free PMC article.
-
Sulfur Metabolism Under Stress.Antioxid Redox Signal. 2020 Dec 1;33(16):1158-1173. doi: 10.1089/ars.2020.8151. Epub 2020 Aug 14. Antioxid Redox Signal. 2020. PMID: 32799544 Free PMC article.
-
Clostridium sticklandii, a specialist in amino acid degradation:revisiting its metabolism through its genome sequence.BMC Genomics. 2010 Oct 11;11:555. doi: 10.1186/1471-2164-11-555. BMC Genomics. 2010. PMID: 20937090 Free PMC article.
-
Structural and kinetic analysis of free methionine-R-sulfoxide reductase from Staphylococcus aureus: conformational changes during catalysis and implications for the catalytic and inhibitory mechanisms.J Biol Chem. 2010 Aug 6;285(32):25044-52. doi: 10.1074/jbc.M110.103119. Epub 2010 May 25. J Biol Chem. 2010. PMID: 20504774 Free PMC article.
References
-
- Collins A, Harrington V. Repair of oxidative DNA damage: assessing its contribution to cancer prevention. Mutagenesis. 2002;17:489–493. - PubMed
-
- Loft S, Poulsen HE. Cancer risk and oxidative DNA damage in man. J Mol Med. 1996;74:297–312. - PubMed
-
- Nair U, Bartsch H, Nair J. Lipid peroxidation-induced DNA damage in cancer-prone inflammatory diseases: a review of published adduct types and levels in humans. Free Radic Biol Med. 2007;43:1109–1120. - PubMed
-
- Rikans LE, Hornbrook KR. Lipid peroxidation, antioxidant protection and aging. Biochim Biophys Acta. 1997;1362:116–127. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

