Bioelectric mechanisms in regeneration: Unique aspects and future perspectives
- PMID: 19406249
- PMCID: PMC2706303
- DOI: 10.1016/j.semcdb.2009.04.013
Bioelectric mechanisms in regeneration: Unique aspects and future perspectives
Abstract
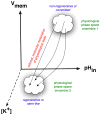
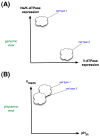
Regenerative biology has focused largely on chemical factors and transcriptional networks. However, endogenous ion flows serve as key epigenetic regulators of cell behavior. Bioelectric signaling involves feedback loops, long-range communication, polarity, and information transfer over multiple size scales. Understanding the roles of endogenous voltage gradients, ion flows, and electric fields will contribute to the basic understanding of numerous morphogenetic processes and the means by which they can robustly restore pattern after perturbation. By learning to modulate the bioelectrical signals that control cell proliferation, migration, and differentiation, we gain a powerful set of new techniques with which to manipulate growth and patterning in biomedical contexts. This chapter reviews the unique properties of bioelectric signaling, surveys molecular strategies and reagents for its investigation, and discusses the opportunities made available for regenerative medicine.
Figures
Comment in
-
Regeneration: Recent advances, major puzzles, and biomedical opportunities.Semin Cell Dev Biol. 2009 Jul;20(5):515-6. doi: 10.1016/j.semcdb.2009.04.011. Epub 2009 May 3. Semin Cell Dev Biol. 2009. PMID: 19398032 No abstract available.
Similar articles
-
Regulation of cell behavior and tissue patterning by bioelectrical signals: challenges and opportunities for biomedical engineering.Annu Rev Biomed Eng. 2012;14:295-323. doi: 10.1146/annurev-bioeng-071811-150114. Annu Rev Biomed Eng. 2012. PMID: 22809139 Free PMC article. Review.
-
Molecular bioelectricity in developmental biology: new tools and recent discoveries: control of cell behavior and pattern formation by transmembrane potential gradients.Bioessays. 2012 Mar;34(3):205-17. doi: 10.1002/bies.201100136. Epub 2012 Jan 11. Bioessays. 2012. PMID: 22237730 Free PMC article. Review.
-
Large-scale biophysics: ion flows and regeneration.Trends Cell Biol. 2007 Jun;17(6):261-70. doi: 10.1016/j.tcb.2007.04.007. Epub 2007 May 10. Trends Cell Biol. 2007. PMID: 17498955 Review.
-
Endogenous bioelectrical networks store non-genetic patterning information during development and regeneration.J Physiol. 2014 Jun 1;592(11):2295-305. doi: 10.1113/jphysiol.2014.271940. J Physiol. 2014. PMID: 24882814 Free PMC article. Review.
-
Transducing bioelectric signals into epigenetic pathways during tadpole tail regeneration.Anat Rec (Hoboken). 2012 Oct;295(10):1541-51. doi: 10.1002/ar.22495. Epub 2012 Aug 29. Anat Rec (Hoboken). 2012. PMID: 22933452 Free PMC article. Review.
Cited by
-
Bioelectric regulation of innate immune system function in regenerating and intact Xenopus laevis.NPJ Regen Med. 2017 May 26;2:15. doi: 10.1038/s41536-017-0019-y. eCollection 2017. NPJ Regen Med. 2017. PMID: 29302351 Free PMC article.
-
Light-activation of the Archaerhodopsin H(+)-pump reverses age-dependent loss of vertebrate regeneration: sparking system-level controls in vivo.Biol Open. 2013 Mar 15;2(3):306-13. doi: 10.1242/bio.20133665. Epub 2013 Jan 17. Biol Open. 2013. PMID: 23519324 Free PMC article.
-
The cell biology of regeneration.J Cell Biol. 2012 Mar 5;196(5):553-62. doi: 10.1083/jcb.201105099. J Cell Biol. 2012. PMID: 22391035 Free PMC article. Review.
-
Transmembrane voltage potential is an essential cellular parameter for the detection and control of tumor development in a Xenopus model.Dis Model Mech. 2013 May;6(3):595-607. doi: 10.1242/dmm.010835. Epub 2013 Feb 1. Dis Model Mech. 2013. PMID: 23471912 Free PMC article.
-
Physical stimuli-emitting scaffolds: The role of piezoelectricity in tissue regeneration.Mater Today Bio. 2023 Jul 20;22:100740. doi: 10.1016/j.mtbio.2023.100740. eCollection 2023 Oct. Mater Today Bio. 2023. PMID: 37521523 Free PMC article. Review.
References
-
- Borgens RB. The role of natural and applied electric fields in neuronal regeneration and development. Progress in Clinical & Biological Research. 1986;210:239–50. - PubMed
-
- Borgens RB, Blight AR, Murphy DJ. Axonal regeneration in spinal cord injury: a perspective and new technique. Journal of Comparative Neurology. 1986;250:157–67. - PubMed
-
- Borgens RB, Blight AR, McGinnis ME. Functional recovery after spinal cord hemisection in guinea pigs: the effects of applied electric fields. J Comp Neurol. 1990;296:634–53. - PubMed
-
- Bentrup F, Sandan T, Jaffe L. Induction of Polarity in Fucus Eggs by Potassium Ion Gradients. Protoplasma. 1967;64:254.
-
- Novák B, Bentrup FW. An electrophysiological study of regeneration in Acetabularia Mediterranea. Planta. 1972;108:227–44. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

