The role of neurosteroids in the pathophysiology and treatment of catamenial epilepsy
- PMID: 19406620
- PMCID: PMC2696558
- DOI: 10.1016/j.eplepsyres.2009.02.017
The role of neurosteroids in the pathophysiology and treatment of catamenial epilepsy
Abstract
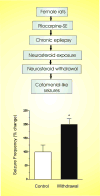
Catamenial epilepsy is a multifaceted neuroendocrine condition in which seizures are clustered around specific points in the menstrual cycle, most often around perimenstrual or periovulatory period. Generally, a twofold or greater increase in seizure frequency during a particular phase of the menstrual cycle could be considered as catamenial epilepsy. Based on this criteria, recent clinical studies indicate that catamenial epilepsy affects 31-60% of the women with epilepsy. Three types of catamenial seizures (perimenstrual, periovulatory and inadequate luteal) have been identified. However, there is no specific drug available today for catamenial epilepsy, which has not been successfully treated with conventional antiepileptic drugs. Elucidation of the pathophysiology of catamenial epilepsy is a prerequisite to develop specific targeted approaches for treatment or prevention of the disorder. Cyclical changes in the circulating levels of estrogens and progesterone play a central role in the development of catamenial epilepsy. There is emerging evidence that endogenous neurosteroids with anticonvulsant or proconvulsant effects could play a critical role in catamenial epilepsy. It is thought that perimenstrual catamenial epilepsy is associated with the withdrawal of anticonvulsant neurosteroids. Progesterone and other hormonal agents have been shown in limited trials to be moderately effective in catamenial epilepsy, but may cause endocrine side effects. Synthetic neurosteroids, which enhance the tonic GABA-A receptor function, might provide an effective approach for the catamenial epilepsy therapy without producing hormonal side effects.
Figures



References
-
- Abassi F, Krumholz A, Kittner SJ, Langenberg P. Effects of menopause on seizures in women with epilepsy. Epilepsia. 1999;42:205–210. - PubMed
-
- Amado D, Cavalheiro EA. Hormonal and gestational parameters in female rats submitted to the pilocarpine model of epilepsy. Epilepsy Res. 1998;32:266–274. - PubMed
-
- Aird RB. The effect of desoxycorticosterone in epilepsy. J Nerv Ment Dis. 1944;99:501–510.
-
- Aird RB, Gordan GS. Anticonvulsive properties of desoxycorticosterone. JAMA. 1951;145:715–719. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

