Longitudinal community plasma HIV-1 RNA concentrations and incidence of HIV-1 among injecting drug users: prospective cohort study
- PMID: 19406887
- PMCID: PMC2675696
- DOI: 10.1136/bmj.b1649
Longitudinal community plasma HIV-1 RNA concentrations and incidence of HIV-1 among injecting drug users: prospective cohort study
Abstract
Objective: To examine the relation between plasma HIV-1 RNA concentrations in the community and HIV incidence among injecting drug users.
Design: Prospective cohort study.
Setting: Inner city community in Vancouver, Canada.
Participants: Injecting drug users, with and without HIV, followed up every six months between 1 May 1996 and 30 June 2007.
Main outcome measures: Estimated community plasma HIV-1 RNA in the six months before each HIV negative participant's follow-up visit. Associated HIV incidence.
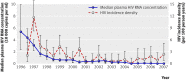
Results: Among 622 injecting drug users with HIV, 12 435 measurements of plasma HIV-1 RNA were obtained. Among 1429 injecting drug users without HIV, there were 155 HIV seroconversions, resulting in an incidence density of 2.49 (95% confidence interval 2.09 to 2.88) per 100 person years. In a Cox model that adjusted for unsafe sexual behaviours and sharing used syringes, the estimated community plasma HIV-1 RNA concentration remained independently associated with the time to HIV seroconversion (hazard ratio 3.32 (1.82 to 6.08, P<0.001), per log(10) increase). When the follow-up period was limited to observations after 1 January 1988 (when the median plasma HIV RNA concentration was <20 000 copies/ml), the median viral load was no longer statistically associated with HIV incidence (1.70 (0.79 to 3.67, P=0.175), per log(10) increase).
Conclusions: A longitudinal measure of community plasma HIV-1 RNA concentration was correlated with the community HIV incidence rate and predicted HIV incidence independent of unsafe sexual behaviours and sharing used syringes. If these findings are confirmed, they could help to inform both HIV prevention and treatment interventions.
Conflict of interest statement
Competing interests: RSH has received funding for research and continuing medical education programmes from pharmaceutical companies, including Abbott, Boehringer Ingelheim, and GlaxoSmithKline. JSGM has received educational grants from, served as an ad hoc adviser to, or spoken at various events sponsored by Abbott Laboratories, Agouron Pharmaceuticals, Boehringer Ingelheim Pharmaceuticals, Borean Pharma AS, Bristol-Myers Squibb, DuPont Pharma, Gilead Sciences, GlaxoSmithKline, Hoffmann-La Roche, Immune Response Corporation, Incyte, Janssen-Ortho, Kucera Pharmaceutical Company, Merck Frosst Laboratories, Pfizer Canada, Sanofi Pasteur, Shire Biochem, Tibotec Pharmaceuticals, and Trimeris.
Ethical approval: The research was approved by the University of British Columbia’s research ethics board at St Paul’s Hospital.
Figures
References
-
- Palella FJ Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med 1998;338:853-60. - PubMed
-
- Hammer SM, Squires KE, Hughes MD, Grimes JM, Demeter LM, Currier JS, et al. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. AIDS Clinical Trials Group 320 Study Team. N Engl J Med 1997;337:725-33. - PubMed
-
- Blower SM, Gershengorn HB, Grant RM. A tale of two futures: HIV and antiretroviral therapy in San Francisco. Science 2000;287:650-4. - PubMed
-
- Velasco-Hernandez JX, Gershengorn HB, Blower SM. Could widespread use of combination antiretroviral therapy eradicate HIV epidemics? Lancet Infect Dis 2002;2:487-93. - PubMed
-
- Cohen MS, Gay C, Kashuba AD, Blower S, Paxton L. Narrative review: antiretroviral therapy to prevent the sexual transmission of HIV-1. Ann Intern Med 2007;146:591-601. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
