Loss of TIMP3 enhances interstitial nephritis and fibrosis
- PMID: 19406980
- PMCID: PMC2689897
- DOI: 10.1681/ASN.2008050492
Loss of TIMP3 enhances interstitial nephritis and fibrosis
Abstract
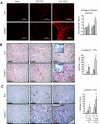
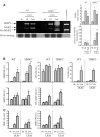
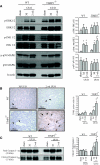
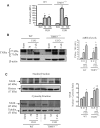
The balance of matrix metalloproteinases (MMPs) and tissue inhibitors of matrix metalloproteinases (TIMPs) determines the integrity of the extracellular matrix. TIMP3 is the most highly expressed tissue inhibitor of metalloproteinase (TIMP) in the kidney, but its function in renal disease is incompletely understood. In this study, TIMP3-/- mice demonstrated an age-dependent chronic tubulointerstitial fibrosis. After unilateral ureteral obstruction (UUO), young TIMP3-/- mice exhibited increased renal injury (tubular atrophy, cortical and medullary thinning, and vascular damage) compared with wild-type mice. In addition, TIMP3-/- mice had greater interstitial fibrosis; increased synthesis and deposition of type I collagen; increased activation of fibroblasts; enhanced apoptosis; and greater activation of MMP2, but not MMP9, after UUO. TIMP3 deficiency also led to accelerated processing of TNFalpha, demonstrated by significantly higher TACE activity and greater soluble TNFalpha levels by 3 d after UUO. The additional deletion of TNFalpha markedly reduced inflammation, apoptosis, and induction of a number of MMPs. Moreover, inhibition of MMPs in TIMP3-/-/TNFalpha-/- mice further abrogated postobstructive injury and prevented tubulointerestitial fibrosis. In humans, TIMP3 expression increased in the renal arteries and proximal tubules of subjects with diabetic nephropathy or chronic allograft nephropathy. Taken together, these results provide evidence that TIMP3 is an important mediator of kidney injury, and regulating its activity may have therapeutic benefit for patients with kidney disease.
Figures




References
-
- Remuzzi G, Bertani T: Pathophysiology of progressive nephropathies. N Engl J Med 339: 1448–1456, 1998 - PubMed
-
- Eddy AA: Molecular basis of renal fibrosis. Pediatr Nephrol 15: 290–301, 2000 - PubMed
-
- Moe OW: Kidney stones: Pathophysiology and medical management. Lancet 367: 333–344, 2006 - PubMed
-
- Nuttall RK, Sampieri CL, Pennington CJ, Gill SE, Schultz GA, Edwards DR: Expression analysis of the entire MMP and TIMP gene families during mouse tissue development. FEBS Lett 563: 129–134, 2004 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

