Human neutrophil peptides and phagocytic deficiency in bronchiectatic lungs
- PMID: 19406984
- PMCID: PMC2714819
- DOI: 10.1164/rccm.200808-1250OC
Human neutrophil peptides and phagocytic deficiency in bronchiectatic lungs
Abstract
Rationale: A well-known clinical paradox is that severe bacterial infections persist in the lungs of patients with cystic fibrosis (CF) despite the abundance of polymorphonuclear neutrophils (PMN) and the presence of a high concentration of human neutrophil peptides (HNP), both of which are expected to kill the bacteria but fail to do so. The mechanisms remain unknown.
Objectives: This study examined several possible mechanisms to understand this paradox.
Methods: PMN were isolated from sputum and blood of subjects with and without CF or non-CF bronchiectasis for phagocytic assays. HNP isolated from patients with CF were used to stimulate healthy PMN followed by phagocytic tests.
Measurements and main results: PMN isolated from the sputum of the bronchiectatic patients display defective phagocytosis that correlated with high concentrations of HNP in the lung. When healthy PMN were incubated with HNP, decreased phagocytic capacity was observed in association with depressed surface Fc gamma RIII, actin-filament remodeling, enhanced intracellular Ca(2+), and degranulation. Treatment of PMN with an intracellular Ca(2+) blocker or alpha1-proteinase inhibitor to attenuate the activity of HNP largely prevented the HNP-induced phagocytic deficiency. Intratracheal instillation of HNP in Pallid mice (genetically deficient in alpha1-proteinase inhibitor) resulted in a greater PMN lung infiltration and phagocytic deficiency compared with wild-type mice.
Conclusions: HNP or PMN alone exert antimicrobial ability, which was lost as a result of their interaction. These effects of HNP may help explain the clinical paradox seen in patients with inflammatory lung diseases, suggesting HNP as a novel target for clinical therapy.
Figures


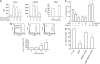
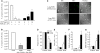
References
-
- Elston C, Geddes D. Inflammation in cystic fibrosis–when and why? Friend or foe? Semin Respir Crit Care Med 2007;28:286–294. - PubMed
-
- Terheggen-Lagro SW, Rijkers GT, van der Ent CK. The role of airway epithelium and blood neutrophils in the inflammatory response in cystic fibrosis. J Cyst Fibros 2005;4:15–23. - PubMed
-
- Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol 2003;3:710–720. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

