p38 MAPK is a major regulator of MafA protein stability under oxidative stress
- PMID: 19407223
- PMCID: PMC2718751
- DOI: 10.1210/me.2008-0482
p38 MAPK is a major regulator of MafA protein stability under oxidative stress
Abstract
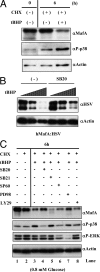
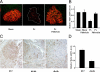
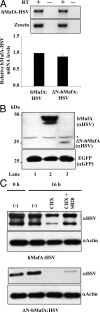
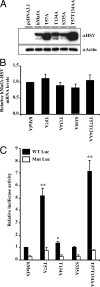
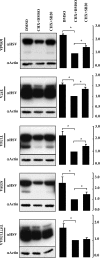
Mammalian MafA/RIPE3b1 is an important glucose-responsive transcription factor that regulates function, maturation, and survival of beta-cells. Increased expression of MafA results in improved glucose-stimulated insulin secretion and beta-cell function. Because MafA is a highly phosphorylated protein, we examined whether regulating activity of protein kinases can increase MafA expression by enhancing its stability. We demonstrate that MafA protein stability in MIN6 cells and isolated mouse islets is regulated by both p38 MAPK and glycogen synthase kinase 3. Inhibiting p38 MAPK enhanced MafA stability in cells grown under both low and high concentrations of glucose. We also show that the N-terminal domain of MafA plays a major role in p38 MAPK-mediated degradation; simultaneous mutation of both threonines 57 and 134 into alanines in MafA was sufficient to prevent this degradation. Under oxidative stress, a condition detrimental to beta-cell function, a decrease in MafA stability was associated with a concomitant increase in active p38 MAPK. Interestingly, inhibiting p38 MAPK but not glycogen synthase kinase 3 prevented oxidative stress-dependent degradation of MafA. These results suggest that the p38 MAPK pathway may represent a common mechanism for regulating MafA levels under oxidative stress and basal and stimulatory glucose concentrations. Therefore, preventing p38 MAPK-mediated degradation of MafA represents a novel approach to improve beta-cell function.
Figures

References
-
- Ohlsson H, Thor S, Edlund T 1991 Novel insulin promoter- and enhancer-binding proteins that discriminate between pancreatic α- and β-cells. Mol Endocrinol 5:897–904 - PubMed
-
- Shieh SY, Tsai MJ 1991 Cell-specific and ubiquitous factors are responsible for the enhancer activity of the rat insulin II gene. J Biol Chem 266:16708–16714 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

