Hypoxic preconditioning enhances the benefit of cardiac progenitor cell therapy for treatment of myocardial infarction by inducing CXCR4 expression
- PMID: 19407239
- PMCID: PMC2756190
- DOI: 10.1161/CIRCRESAHA.109.197723
Hypoxic preconditioning enhances the benefit of cardiac progenitor cell therapy for treatment of myocardial infarction by inducing CXCR4 expression
Abstract
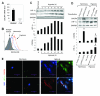
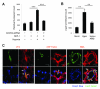
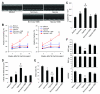
Myocardial infarction rapidly depletes the endogenous cardiac progenitor cell pool, and the inefficient recruitment of exogenously administered progenitor cells limits the effectiveness of cardiac cell therapy. Recent reports indicate that interactions between the CXC chemokine stromal cell-derived factor 1 and its receptor CXC chemokine receptor 4 (CXCR4) critically mediate the ischemia-induced recruitment of bone marrow-derived circulating stem/progenitor cells, but the expression of CXCR4 in cardiac progenitor cells is very low. Here, we studied the influence of hypoxia on CXCR4 expression in cardiac progenitor cells, on the recruitment of intravenously administered cells to ischemic heart tissue, and on the preservation of heart function in a murine myocardial infarction model. We found that hypoxic preconditioning increased CXCR4 expression in CLK (cardiosphere-derived, Lin(-)c-kit(+) progenitor) cells and markedly augmented CLK cell migration (in vitro) and recruitment (in vivo) to the ischemic myocardium. Four weeks after surgically induced myocardial infarction, infarct size and heart function were significantly better in mice administered hypoxia-preconditioned CLK cells than in mice treated with cells cultured under normoxic conditions. Furthermore, these effects were largely abolished by the addition of a CXCR4 inhibitor, indicating that the benefits of hypoxic preconditioning are mediated by the stromal cell-derived factor 1/CXCR4 axis, and that therapies targeting this axis may enhance cardiac-progenitor cell-based regenerative therapy.
Figures

Comment in
-
Importance of the SDF-1:CXCR4 axis in myocardial repair.Circ Res. 2009 May 22;104(10):1133-5. doi: 10.1161/CIRCRESAHA.109.198929. Circ Res. 2009. PMID: 19461103 Free PMC article. No abstract available.
References
-
- Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, Haase N, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O’Donnell CJ, Roger V, Rumsfeld J, Sorlie P, Steinberger J, Thom T, Wasserthiel-Smoller S, Hong Y. Heart disease and stroke statistics--2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–171. - PubMed
-
- Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. - PubMed
-
- Martin CM, Meeson AP, Robertson SM, Hawke TJ, Richardson JA, Bates S, Goetsch SC, Gallardo TD, Garry DJ. Persistent expression of the ATP-binding cassette transporter, Abcg2, identifies cardiac SP cells in the developing and adult heart. Dev Biol. 2004;265:262–275. - PubMed
-
- Matsuura K, Nagai T, Nishigaki N, Oyama T, Nishi J, Wada H, Sano M, Toko H, Akazawa H, Sato T, Nakaya H, Kasanuki H, Komuro I. Adult cardiac Sca-1-positive cells differentiate into beating cardiomyocytes. J Biol Chem. 2004;279:11384–11391. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

