Safety and adequacy of a semi-elemental formula for children with gastro-intestinal disease
- PMID: 19408097
- PMCID: PMC2839472
- DOI: 10.1007/s00726-009-0298-8
Safety and adequacy of a semi-elemental formula for children with gastro-intestinal disease
Abstract
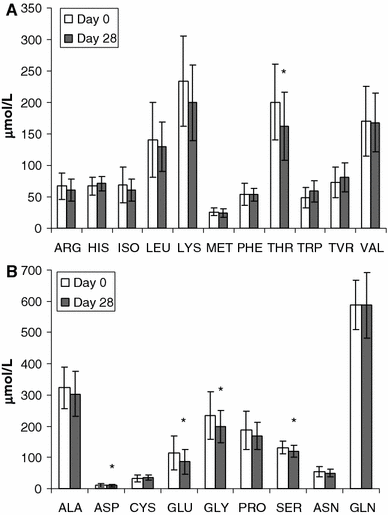
A prospective, open trial was conducted to evaluate the nutritional adequacy of a semi-elemental diet in 47 children with functional gastro-intestinal disorders. Nutritional adequacy was assessed based on growth relative to Euro-growth standards for body mass index (BMI)-for-age z-scores and evaluations of blood parameters. Twenty-five patients completed the study. In total, 533 l of "New-Alfare" was consumed during 775 trial-days. The mean intake per infant was 85.8 +/- 26.8 kcal/kg/day or 122.5 +/- 38.3 ml/kg/day. Weight and length evolution during the 4 weeks trial were within normal range. The mean BMI-for-age z-score (P < 0.05) and albumin concentration (P < 0.01) increased significantly after 4 weeks. Plasma threonine concentration decreased significantly (P = 0.01) and the tryptophan concentration increased (P = 0.06). No adverse events related to the study formula were reported. These results show that "New Alfaré" is safe and nutritionally adequate for pediatric patients with gastro-intestinal disease.
Figures
Similar articles
-
Associations of hyperosmolar medications administered via nasogastric or nasoduodenal tubes and feeding adequacy, food intolerance and gastrointestinal complications amongst critically ill patients: A retrospective study.Clin Nutr ESPEN. 2018 Jun;25:78-86. doi: 10.1016/j.clnesp.2018.04.001. Epub 2018 Apr 17. Clin Nutr ESPEN. 2018. PMID: 29779822
-
Use of standard enteral formula versus enteric formula with prebiotic content in nutrition therapy: A randomized controlled study among neuro-critical care patients.Clin Nutr ESPEN. 2018 Jun;25:26-36. doi: 10.1016/j.clnesp.2018.03.123. Epub 2018 Mar 30. Clin Nutr ESPEN. 2018. PMID: 29779815 Clinical Trial.
-
[Clinical analysis of enteral nutrition in 47 children].Zhonghua Er Ke Za Zhi. 2016 Jul;54(7):500-3. doi: 10.3760/cma.j.issn.0578-1310.2016.07.005. Zhonghua Er Ke Za Zhi. 2016. PMID: 27412739 Chinese.
-
Nutritional modulation of neonatal outcomes.AACN Clin Issues. 2004 Jan-Mar;15(1):83-96. doi: 10.1097/00044067-200401000-00007. AACN Clin Issues. 2004. PMID: 14767367 Review.
-
Primary and secondary prevention of atopic diseases in children.Curr Probl Dermatol. 1999;28:173-93. doi: 10.1159/000060591. Curr Probl Dermatol. 1999. PMID: 10374064 Review. No abstract available.
References
-
- Darling PB, Dunn M, Sarwar G, Brookes S, Ball RO, Pencharz PB. Threonine kinetics in preterm infants fed their mothers’ milk or formula with various ratios of whey to casein. Am J Clin Nutr. 1999;69:105–114. - PubMed
-
- Exl BM, Deland U, Secretin MC, Preysch U, Wall M, Shmerling DH. Improved general health status in an unselected infant population following an allergen-reduced dietary intervention programme: the ZUFF-STUDY-PROGRAMME. Part II: infant growth and health status to age 6 months. ZUg-FrauenFeld. Eur J Nutr. 2000;39:145–156. doi: 10.1007/s003940070018. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical