CD4+ lymphocytes modulate prostate cancer progression in mice
- PMID: 19408303
- PMCID: PMC4400664
- DOI: 10.1002/ijc.24452
CD4+ lymphocytes modulate prostate cancer progression in mice
Abstract
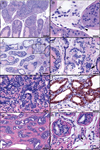
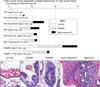
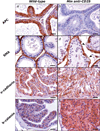
Chronic inflammation contributes to the development of prostate cancer in humans. Here, we show that male Apc(Min/+) mice also develop prostate carcinoma with increasing age, mimicking that seen in humans in their 5th or 6th decade of life. Proinflammatory cytokines were significantly linked with cancer and increasing age in our mouse model; however, prostate and bowel tissues lacked evidence of inflammatory cell infiltrates other than mast cells. Lymphocytes protected against cancer, and protection from prostate cancer resided in antiinflammatory CD4(+)CD25(+) regulatory (T(REG)) cells that downregulated inflammatory cytokines. Supplementation with syngeneic T(REG) cells collected from wild-type mice reduced the levels of interleukin (IL)-6 (p < 0.05) and IL-9 (p < 0.001) and lowered prostate cancer risk (p < 0.05). Depletion of CD25(+) cells in 2-month-old animals increased the expression of IL-6 (p < 0.005) within prostate and increased the frequency of high-grade prostatic intraepithelial neoplasia (p < 0.05) and microinvasive prostatic carcinoma (p < 0.05) in dorsolateral prostate. Depletion of CD25(+) cells in young animals also increased the frequency of intestinal cancer in Min mice. Taken together, chronically elevated proinflammatory cytokines promoted carcinoma in Apc(Min/+) mice. T(REG) lymphocytes downregulated inflammation-associated carcinogenic processes and contributed to immune and epithelial homeostasis.
Figures



Similar articles
-
Roles for inflammation and regulatory T cells in colon cancer.Toxicol Pathol. 2010 Jan;38(1):76-87. doi: 10.1177/0192623309354110. Epub 2009 Dec 17. Toxicol Pathol. 2010. PMID: 20019355 Free PMC article. Review.
-
Unifying roles for regulatory T cells and inflammation in cancer.Int J Cancer. 2010 Apr 1;126(7):1651-65. doi: 10.1002/ijc.24923. Int J Cancer. 2010. PMID: 19795459 Free PMC article.
-
Ablation of IL-17A abrogates progression of spontaneous intestinal tumorigenesis.Proc Natl Acad Sci U S A. 2010 Mar 23;107(12):5540-4. doi: 10.1073/pnas.0912675107. Epub 2010 Mar 8. Proc Natl Acad Sci U S A. 2010. PMID: 20212110 Free PMC article.
-
CD4+ T helper 17 cell response of aged mice promotes prostate cancer cell migration and invasion.Prostate. 2020 Jul;80(10):764-776. doi: 10.1002/pros.23990. Epub 2020 May 1. Prostate. 2020. PMID: 32356608 Free PMC article.
-
Colitis-associated cancer: the role of T cells in tumor development.Semin Immunopathol. 2009 Jul;31(2):249-56. doi: 10.1007/s00281-009-0161-8. Epub 2009 Jun 3. Semin Immunopathol. 2009. PMID: 19495757 Review.
Cited by
-
Cytokines expression levels from tissue, plasma or serum as promising clinical biomarkers in adenocarcinoma of the prostate: a systematic review of recent findings.Ann Transl Med. 2019 Jun;7(11):245. doi: 10.21037/atm.2019.05.31. Ann Transl Med. 2019. PMID: 31317015 Free PMC article. Review.
-
Animal models relevant to human prostate carcinogenesis underlining the critical implication of prostatic stem/progenitor cells.Biochim Biophys Acta. 2011 Aug;1816(1):25-37. doi: 10.1016/j.bbcan.2011.03.001. Epub 2011 Mar 17. Biochim Biophys Acta. 2011. PMID: 21396984 Free PMC article. Review.
-
YYFZBJS ameliorates colorectal cancer progression in ApcMin/+ mice by remodeling gut microbiota and inhibiting regulatory T-cell generation.Cell Commun Signal. 2020 Jul 16;18(1):113. doi: 10.1186/s12964-020-00596-9. Cell Commun Signal. 2020. PMID: 32677955 Free PMC article.
-
Roles for inflammation and regulatory T cells in colon cancer.Toxicol Pathol. 2010 Jan;38(1):76-87. doi: 10.1177/0192623309354110. Epub 2009 Dec 17. Toxicol Pathol. 2010. PMID: 20019355 Free PMC article. Review.
-
Treatment combining RU486 and Ad5IL-12 vector attenuates the growth of experimentally formed prostate tumors and induces changes in the sentinel lymph nodes of mice.J Transl Med. 2010 Oct 14;8:98. doi: 10.1186/1479-5876-8-98. J Transl Med. 2010. PMID: 20946663 Free PMC article.
References
-
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–130. - PubMed
-
- Sakr WA, Grignon DJ, Crissman JD, Heilbrun LK, Cassin BJ, Pontes JJ, Haas GP. High grade prostatic intraepithelial neoplasia (HGPIN) and prostatic adenocarcinoma between the ages of 20–69: an autopsy study of 249 cases. In Vivo. 1994;8:439–443. - PubMed
-
- Nelson WG, De Marzo AM, Isaacs WB. Prostate cancer. N Engl J Med. 2003;349:366–381. - PubMed
-
- Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

