Magnetic resonance imaging of Huntington's disease: preparing for clinical trials
- PMID: 19409230
- PMCID: PMC2771270
- DOI: 10.1016/j.neuroscience.2009.01.045
Magnetic resonance imaging of Huntington's disease: preparing for clinical trials
Abstract
The known genetic mutation causing Huntington's disease (HD) makes this disease an important model to study links between gene and brain function. An autosomal dominant family history and the availability of a sensitive and specific genetic test allow pre-clinical diagnosis many years before the onset of any typical clinical signs. This review summarizes recent magnetic resonance imaging (MRI)-based findings in HD with a focus on the requirements if imaging is to be used in treatment trials. Despite its monogenetic cause, HD presents with a range of clinical manifestations, not explained by variation in the number of CAG repeats in the affected population. Neuroimaging studies have revealed a complex pattern of structural and functional changes affecting widespread cortical and subcortical regions far beyond the confines of the striatal degeneration that characterizes this disorder. Besides striatal dysfunction, functional imaging studies have reported a variable pattern of increased and decreased activation in cortical regions in both pre-clinical and clinically manifest HD-gene mutation carriers. Beyond regional brain activation changes, evidence from functional and diffusion-weighted MRI further suggests disrupted connectivity between corticocortical and corticostriatal areas. However, substantial inconsistencies with respect to structural and functional changes have been reported in a number of studies. Possible explanations include methodological factors and differences in study samples. There may also be biological explanations but these are poorly characterized and understood at present. Additional insights into this phenotypic variability derived from study of mouse models are presented to explore this phenomenon.
Figures

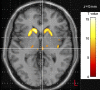
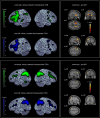
References
-
- Ashburner J., Friston K.J. Voxel-based morphometry—the methods. Neuroimage. 2000;11:805–821. - PubMed
-
- Aylward E.H. Change in MRI striatal volumes as a biomarker in preclinical Huntington's disease. Brain Res Bull. 2007;72:152–158. - PubMed
-
- Aylward E.H., Anderson N.B., Bylsma F.W., Wagster M.V., Barta P.E., Sherr M., Feeney J., Davis A., Rosenblatt A., Pearlson G.D., Ross C.A. Frontal lobe volume in patients with Huntington's disease. Neurology. 1998;50:252–258. - PubMed
-
- Aylward E.H., Brandt J., Codori A.M., Mangus R.S., Barta P.E., Harris G.J. Reduced basal ganglia volume associated with the gene for Huntington's disease in asymptomatic at-risk persons. Neurology. 1994;44:823–828. - PubMed
-
- Aylward E.H., Codori A.M., Barta P.E., Pearlson G.D., Harris G.J., Brandt J. Basal ganglia volume and proximity to onset in presymptomatic Huntington disease. Arch Neurol. 1996;53:1293–1296. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

