Circumventricular organs: a novel site of neural stem cells in the adult brain
- PMID: 19409493
- PMCID: PMC2697272
- DOI: 10.1016/j.mcn.2009.04.007
Circumventricular organs: a novel site of neural stem cells in the adult brain
Abstract
Neurogenesis in the adult mammalian nervous system is now well established in the subventricular zone of the anterolateral ventricle and subgranular zone of the hippocampus. In these regions, neurons are thought to arise from neural stem cells, identified by their expression of specific intermediate filament proteins (nestin, vimentin, GFAP) and transcription factors (Sox2). In the present study, we show that in adult rat and mouse, the circumventricular organs (CVOs) are rich in nestin+, GFAP+, vimentin+ cells which express Sox2 and the cell cycle-regulating protein Ki67. In culture, these cells proliferate as neurospheres and express neuronal (doublecortin+, beta-tubulin III+) and glial (S100beta+, GFAP+, RIP+) phenotypic traits. Further, our in vivo studies using bromodeoxyuridine show that CVO cells proliferate and undergo constitutive neurogenesis and gliogenesis. These findings suggest that CVOs may constitute a heretofore unknown source of stem/progenitor cells, capable of giving rise to new neurons and/or glia in the adult brain.
Figures






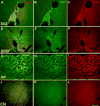

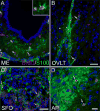

References
-
- Alvarez-Buylla A, Lim DA. For the long run: Maintaining germinal niches in the adult brain. Neuron. 2004;41:683–686. - PubMed
-
- Arochena M, Anadon R, Diaz-Regueira SM. Development of vimentin and glial fibrillary acidic protein immunoreactivities in the brain of gray mullet (chelon labrosus), an advanced teleost. J Comp Neurol. 2004;469:413–436. - PubMed
-
- Barry D, McDermott K. Differentiation of radial glia from radial precursor cells and transformation into astrocytes in the developing rat spinal cord. Glia. 2005;50:187–197. - PubMed
-
- Bauer S, Hay M, Amilhon B, Jean A, Moyse E. In vivo neurogenesis in the dorsal vagal complex of the adult rat brainstem. Neuroscience. 2005;130:75–90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

