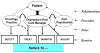
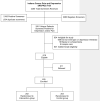
The Indiana Cancer Pain and Depression (INCPAD) trial Design of a telecare management intervention for cancer-related symptoms and baseline characteristics of study participants
- PMID: 19410103
- PMCID: PMC2743872
- DOI: 10.1016/j.genhosppsych.2009.01.007
The Indiana Cancer Pain and Depression (INCPAD) trial Design of a telecare management intervention for cancer-related symptoms and baseline characteristics of study participants
Abstract
Objective: Pain and depression are two of the most prevalent and treatable cancer-related symptoms, each present in at least 20-30% of oncology patients. Both symptoms are frequently either unrecognized or undertreated, however. This article describes a telecare management intervention delivered by a nurse-psychiatrist team that is designed to improve recognition and treatment of pain and depression. The enrolled sample is also described.
Methods: The Indiana Cancer Pain and Depression study is a National Cancer Institute-sponsored randomized clinical trial. Four hundred five patients with cancer-related pain and/or clinically significant depression from 16 urban or rural oncology practices throughout Indiana have been enrolled and randomized to either the intervention group or to a usual-care control group. Intervention patients receive centralized telecare management coupled with automated home-based symptom monitoring. Outcomes will be assessed at 1, 3, 6 and 12 months by research assistants blinded to treatment arms.
Results: Of 4465 patients screened, 2185 (49%) endorsed symptoms of pain or depression. Of screen-positive patients, about one-third were ineligible (most commonly due to pain or depression not meeting severity thresholds or to pain that is not related to cancer). Of the 405 patients enrolled, 32% have depression only, 24% have pain only and 44% have both depression and pain. At baseline, participants reported an average of 16.8 days out of the past 4 weeks during which they were confined to bed or had to reduce their usual activities by > or =50% due to pain or depression. Also, 176 (44%) reported being unable to work due to health reasons.
Conclusions: When completed, the Indiana Cancer Pain and Depression trial will test whether centralized telecare management coupled with automated home-based symptom monitoring improves outcomes in cancer patients with depression and/or pain. Findings will be important for both oncologists and mental health clinicians confronted with oncology patients' depression or pain.
Figures
References
-
- Carr D, Goudas L, Lawrence D, Pirl W, Lau J, DeVine D, et al. Evidence Report/Technology Assessment No. 61 (Prepared by the New England Medical Center Evidence-based Practice Center under contract No 290-97-0019) Rockville, MD: Agency for Healthcare Research and Quality; 2002. Management of cancer symptoms: Pain, depression, and fatigue. AHRQ Publication No. 02-E032. - PMC - PubMed
-
- Bottomley A. Depression in cancer patients: a literature review. Eur J Cancer Care. 1998;7:181–91. - PubMed
-
- Caraceni A, Portenoy RK. An international survey of cancer pain characteristics and syndromes. IASP Task Force on Cancer Pain. International Association for the Study of Pain. Pain. 1999;82:263–74. - PubMed
-
- Portenoy RK, Lesage P. Management of cancer pain. Lancet. 1999;353:1695–700. - PubMed
-
- Given CW, Given B, Azzouz F, Kozachik S, Stommel M. Predictors of pain and fatigue in the year following diagnosis among elderly cancer patients. J Pain Symptom Manage. 2001;21:456–66. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous