HIV enters cells via endocytosis and dynamin-dependent fusion with endosomes
- PMID: 19410541
- PMCID: PMC2696170
- DOI: 10.1016/j.cell.2009.02.046
HIV enters cells via endocytosis and dynamin-dependent fusion with endosomes
Abstract
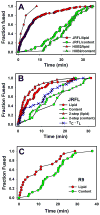
Enveloped viruses that rely on a low pH-dependent step for entry initiate infection by fusing with acidic endosomes, whereas the entry sites for pH-independent viruses, such as HIV-1, have not been defined. These viruses have long been assumed to fuse directly with the plasma membrane. Here we used population-based measurements of the viral content delivery into the cytosol and time-resolved imaging of single viruses to demonstrate that complete HIV-1 fusion occurred in endosomes. In contrast, viral fusion with the plasma membrane did not progress beyond the lipid mixing step. HIV-1 underwent receptor-mediated internalization long before endosomal fusion, thus minimizing the surface exposure of conserved viral epitopes during fusion and reducing the efficacy of inhibitors targeting these epitopes. We also show that, strikingly, endosomal fusion is sensitive to a dynamin inhibitor, dynasore. These findings imply that HIV-1 infects cells via endocytosis and envelope glycoprotein- and dynamin-dependent fusion with intracellular compartments.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures





Comment in
-
HIV Entry Revisited.Cell. 2009 May 1;137(3):402-4. doi: 10.1016/j.cell.2009.04.033. Cell. 2009. PMID: 19410537 Free PMC article.
References
-
- Brandt SM, Mariani R, Holland AU, Hope TJ, Landau NR. Association of chemokine-mediated block to HIV entry with coreceptor internalization. J Biol Chem. 2002;277:17291–17299. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

