CDK inhibitor p18(INK4c) is a downstream target of GATA3 and restrains mammary luminal progenitor cell proliferation and tumorigenesis
- PMID: 19411068
- PMCID: PMC2699569
- DOI: 10.1016/j.ccr.2009.03.004
CDK inhibitor p18(INK4c) is a downstream target of GATA3 and restrains mammary luminal progenitor cell proliferation and tumorigenesis
Abstract
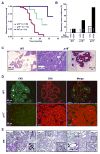
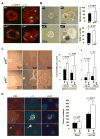
Mammary epithelia are composed of luminal and myoepithelial/basal cells whose neoplastic transformations lead to distinct types of breast cancers with diverse clinical features. We report that mice deficient for the CDK4/6 inhibitor p18(Ink4c) spontaneously develop ER-positive luminal tumors at a high penetrance. Ink4c deletion stimulates luminal progenitor cell proliferation at pubertal age and maintains an expanded luminal progenitor cell population throughout life. We demonstrate that GATA3 binds to and represses INK4C transcription. In human breast cancers, low INK4C and high GATA3 expressions are simultaneously observed in luminal A type tumors and predict a favorable patient outcome. Hence, p18(INK4C) is a downstream target of GATA3, constrains luminal progenitor cell expansion, and suppresses luminal tumorigenesis in the mammary gland.
Figures





References
-
- Althuis MD, Fergenbaum JH, Garcia-Closas M, Brinton LA, Madigan MP, Sherman ME. Etiology of hormone receptor-defined breast cancer: a systematic review of the literature. Cancer Epidemiol Biomarkers Prev. 2004;13:1558–1568. - PubMed
-
- Asselin-Labat ML, Sutherland KD, Barker H, Thomas R, Shackleton M, Forrest NC, Hartley L, Robb L, Grosveld FG, van der Wees J, et al. Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nat Cell Biol. 2007;9:201–209. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

