Homeostatic and stimulus-induced coupling of the L-type Ca2+ channel to the ryanodine receptor in the hippocampal neuron in slices
- PMID: 19411104
- PMCID: PMC2703683
- DOI: 10.1016/j.ceca.2009.03.018
Homeostatic and stimulus-induced coupling of the L-type Ca2+ channel to the ryanodine receptor in the hippocampal neuron in slices
Abstract
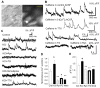
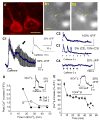
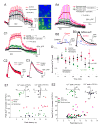
Activity-dependent increase in cytosolic calcium ([Ca(2+)](i)) is a prerequisite for many neuronal functions. We previously reported a strong direct depolarization, independent of glutamate receptors, effectively caused a release of Ca(2+) from ryanodine-sensitive stores and induced the synthesis of endogenous cannabinoids (eCBs) and eCB-mediated responses. However, the cellular mechanism that initiated the depolarization-induced Ca(2+)-release is not completely understood. In the present study, we optically recorded [Ca(2+)](i) from CA1 pyramidal neurons in the hippocampal slice and directly monitored miniature Ca(2+) activities and depolarization-induced Ca(2+) signals in order to determine the source(s) and properties of [Ca(2+)](i)-dynamics that could lead to a release of Ca(2+) from the ryanodine receptor. In the absence of depolarizing stimuli, spontaneously occurring miniature Ca(2+) events were detected from a group of hippocampal neurons. This miniature Ca(2+) event persisted in the nominal Ca(2+)-containing artificial cerebrospinal fluid (ACSF), and increased in frequency in response to the bath-application of caffeine and KCl. In contrast, nimodipine, the antagonist of the L-type Ca(2+) channel (LTCC), a high concentration of ryanodine, the antagonist of the ryanodine receptor (RyR), and thapsigargin (TG) reduced the occurrence of the miniature Ca(2+) events. When a brief puff-application of KCl was given locally to the soma of individual neurons in the presence of glutamate receptor antagonists, these neurons generated a transient increase in the [Ca(2+)](i) in the dendrosomal region. This [Ca(2+)](i)-transient was sensitive to nimodipine, TG, and ryanodine suggesting that the [Ca(2+)](i)-transient was caused primarily by the LTCC-mediated Ca(2+)-influx and a release of Ca(2+) from RyR. We observed little contribution from N- or P/Q-type Ca(2+) channels. The coupling between LTCC and RyR was direct and independent of synaptic activities. Immunohistochemical study revealed a cellular localization of LTCC and RyR in a juxtaposed configuration in the proximal dendrites and soma. We conclude in the hippocampal CA1 neuron that: (1) homeostatic fluctuation of the resting membrane potential may be sufficient to initiate functional coupling between LTCC and RyR; (2) the juxtaposed localization of LTCC and RyR has anatomical advantage of synchronizing a Ca(2+)-release from RyR upon the opening of LTCC; and (3) the synchronized Ca(2+)-release from RyR occurs immediately after the activation of LTCC and determines the peak amplitude of depolarization-induced global increase in dendrosomal [Ca(2+)](i).
Figures

References
-
- Chavis P, Fagni L, Lansman JB, Bockaert J. Functional coupling between ryanodine receptors and L-type calcium channels in neurons. Nature. 1996;382:719–722. - PubMed
-
- Bito H, Deisseroth K, Tsien RW. CREB phosphorylation and dephosphorylation: a Ca and stimulus duration-dependent switch for hippocampal gene expression. Cell. 1996;87:1203–1214. - PubMed
-
- Bouchard R, Pattarini R, Geiger JD. Presence and functional significance of presynaptic ryanodine receptors. Prog Neurobiol. 2003;69:391–418. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

