Glucose-stimulated expression of Txnip is mediated by carbohydrate response element-binding protein, p300, and histone H4 acetylation in pancreatic beta cells
- PMID: 19411249
- PMCID: PMC2719326
- DOI: 10.1074/jbc.M109.010504
Glucose-stimulated expression of Txnip is mediated by carbohydrate response element-binding protein, p300, and histone H4 acetylation in pancreatic beta cells
Abstract
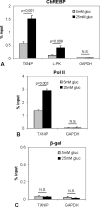
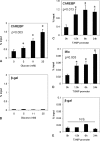
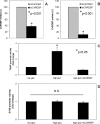
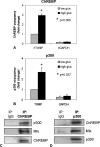
Recently, we identified Txnip (thioredoxin-interacting protein) as a mediator of glucotoxic beta cell death and discovered that lack of Txnip protects against streptozotocin- and obesity-induced diabetes by preventing beta cell apoptosis and preserving endogenous beta cell mass. Txnip has therefore become an attractive target for diabetes therapy, but although we have found that txnip transcription is highly induced by glucose through a unique carbohydrate response element, the factors controlling this effect have remained unknown. Using transient transfection experiments, we now show that overexpression of the carbohydrate response element-binding protein (ChREBP) transactivates the txnip promoter, whereas ChREBP knockdown by small interfering RNA completely blunts glucose-induced txnip transcription. Moreover, chromatin immunoprecipitation demonstrated that glucose leads to a dose- and time-dependent recruitment of ChREBP to the txnip promoter in vivo in INS-1 beta cells as well as human islets. Furthermore, we found that the co-activator and histone acetyltransferase p300 co-immunoprecipitates with ChREBP and also binds to the txnip promoter in response to glucose. Interestingly, this is associated with specific acetylation of histone H4 and recruitment of RNA polymerase II as measured by chromatin immunoprecipitation. Thus, with this study we have identified ChREBP as the transcription factor that mediates glucose-induced txnip expression in human islets and INS-1 beta cells and have characterized the chromatin modification associated with glucose-induced txnip transcription. In addition, the results reveal for the first time that ChREBP interacts with p300. This may explain how ChREBP induces H4 acetylation and sheds new light on glucose-mediated regulation of chromatin structure and transcription.
Figures




References
-
- Junn E., Han S. H., Im J. Y., Yang Y., Cho E. W., Um H. D., Kim D. K., Lee K. W., Han P. L., Rhee S. G., Choi I. ( 2000) J. Immunol. 164, 6287– 6295 - PubMed
-
- Nishiyama A., Masutani H., Nakamura H., Nishinaka Y., Yodoi J. ( 2001) IUBMB Life 52, 29– 33 - PubMed
-
- Wang Y., De Keulenaer G. W., Lee R. T. ( 2002) J. Biol. Chem. 277, 26496– 26500 - PubMed
-
- Minn A. H., Hafele C., Shalev A. ( 2005) Endocrinology 146, 2397– 2405 - PubMed
-
- Eizirik D. L., Kutlu B., Rasschaert J., Darville M., Cardozo A. K. ( 2003) Ann. N.Y. Acad. Sci. 1005, 55– 74 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

