Role of cGMP-dependent protein kinase in regulation of pulmonary vascular smooth muscle cell adhesion and migration: effect of hypoxia
- PMID: 19411288
- PMCID: PMC2711721
- DOI: 10.1152/ajpheart.00077.2008
Role of cGMP-dependent protein kinase in regulation of pulmonary vascular smooth muscle cell adhesion and migration: effect of hypoxia
Abstract
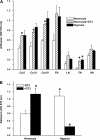
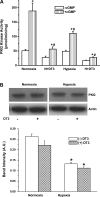
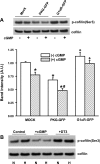
Exposure to prolonged hypoxia can result in pulmonary vascular remodeling and pulmonary hypertension. Hypoxia induces pulmonary vascular smooth muscle cell (PVSMC) proliferation and vascular remodeling by affecting cell adhesion and migration and secretion of extracellular matrix proteins. We previously showed that acute hypoxia decreases cGMP-dependent protein kinase (PKG) activity in PVSMC and that PKG plays a role in maintaining the differentiated contractile phenotype in normoxia. In this study, we investigated the effect of hypoxia on PVSMC adhesion and migration and the role of PKG in these functions. Ovine fetal pulmonary artery SMC were incubated in normoxia (Po(2) approximately 100 Torr) or hypoxia (Po(2) approximately 30-40 Torr) or treated with the PKG inhibitor DT-3 for 24 h in normoxia. To further study the role of PKG in the modulation of adhesion and migration, PVSMC were transiently transfected with a full-length PKG1alpha [PKG-green fluorescent protein (GFP)] or a dominant-negative construct (G1alphaR-GFP). Cell adhesion to extracellular matrix proteins was determined, and integrin-mediated adhesion was assessed by alpha/beta-integrin-mediated cell adhesion array. Exposure to hypoxia (24 h) and pharmacological inhibition of PKG1 by DT-3 significantly promoted adhesion mediated by alpha(4)-, beta(1)-, and alpha(5)beta(1)-integrins to fibronectin, laminin, and tenacin and also resulted in increased cell migration. Likewise, inhibition of PKG by expression of a dominant-negative PKG1alpha construct increased cell adhesion and migration, comparable to that induced by hypoxia. Dynamic actin reorganization associated with integrin-mediated cell adhesion is partly regulated by the actin-binding protein cofilin, the (Ser3) phosphorylation of which inhibits its actin-severing activity. We found that increased PKG expression and activity is associated with decreased cofilin (Ser3) phosphorylation, implying a role for PKG in the modulation of cofilin activity and actin dynamics. Together, these findings identify cGMP/PKG1 signaling as central to the functional differences between PVSMC exposed to normoxia versus hypoxia.
Figures



References
-
- Abedi H, Zachary I. Signaling mechanisms in the regulation of vascular cell migration. Cardiovasc Res 30: 544–556, 1995. - PubMed
-
- Blaschke F, Stawowy P, Goetze S, Hintz O, Grafe M, Kintscher U, Fleck E, Graf K. Hypoxia activates beta1-integrin via ERK 1/2 and p38 MAP kinase in human vascular smooth muscle cells. Biochem Biophys Res Commun 296: 890–896, 2002. - PubMed
-
- Browning DD, McShane M, Marty C, Ye RD. Functional analysis of type 1alpha cGMP-dependent protein kinase using green fluorescent fusion proteins. J Biol Chem 276: 13039–13048, 2001. - PubMed
-
- Chen H, Bernstein BW, Bamburg JR. Regulating actin-filament dynamics in vivo. Trends Biochem Sci 25: 19–23, 2000. - PubMed
-
- Corley KM, Taylor CJ, Lilly B. Hypoxia-inducible factor 1alpha modulates adhesion, migration, and FAK phosphorylation in vascular smooth muscle cells. J Cell Biochem 96: 971–985, 2005. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

