Aberrant cell adhesion molecule expression in human bronchopulmonary sequestration and congenital cystic adenomatoid malformation
- PMID: 19411307
- PMCID: PMC2711815
- DOI: 10.1152/ajplung.90618.2008
Aberrant cell adhesion molecule expression in human bronchopulmonary sequestration and congenital cystic adenomatoid malformation
Abstract
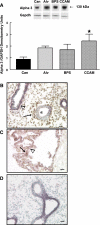
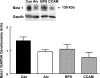
In many organs, integrins and cadherins are partly regulated by Hox genes, but their interactions in airway morphogenesis and congenital lung diseases are unknown. We previously showed that the Hox protein HoxB5 is abnormally increased in bronchopulmonary sequestration (BPS) and congenital cystic adenomatoid malformation (CCAM), congenital lung lesions with abnormal airway branching. We now report on alpha(2)-, alpha(3)-, and beta(1)-integrin and E-cadherin expression in normal human lung and in BPS and CCAM tissue previously shown to have abnormal HoxB5 expression and on the relationship of cell adhesion molecule expression to Hoxb5 regulation. alpha(2)-, alpha(3)-, and beta(1)-integrins and E-cadherin expression in normal human lung and BPS and CCAM were evaluated using Western blot and immunohistochemistry. Fetal mouse lung fibroblasts with Hoxb5-specific siRNA downregulation were evaluated for alpha(2)-integrin protein levels by Western blot. Compared with normal human lung, a previously undetected alpha(2)-integrin isoform potentially lacking essential cytoplasmic sequences was significantly increased in BPS and CCAM, and alpha(2)-integrin spatial and cellular expression was more intense. E-cadherin protein levels were also significantly increased, whereas alpha(3) increased in CCAM compared with canalicular, but not with alveolar, stage lung. beta(1)-integrin levels were unchanged. We conclude that in BPS and CCAM, altered alpha(2)-integrin cytoplasmic signaling contributes to abnormal cellular behavior in these lung lesions. Aberrant cell adhesion molecule and Hox protein regulation are likely part of the mechanism involved in the development of BPS and CCAM.
Figures




References
-
- Achiron R, Hegesh J, Yagel S. Fetal lung lesions: a spectrum of disease. New classification based on pathogenesis, two dimensional and color doppler ultrasound. Ultrasound Obstet Gynecol 24: 107–114, 2004. - PubMed
-
- Achiron R, Zalel Y, Lipitz S, Hegesh J, Mazkereth R, Kuint J, Jacobson J, Yagel S. Fetal lung dysplasia: clinical outcome based on a new classification system. Ultrasound Obstet Gynecol 24: 127–133, 2004. - PubMed
-
- Aulicino MR, Reis ED, Dolgin SE, Unger PD, Shah KD. Intra-abdominal pulmonary sequestration exhibiting congenital cystic adenomatoid malformation: report of a case and review of literature. Arch Pathol Lab Med 118: 1034–1037, 1994. - PubMed
-
- Barr LF, Cambell SE, Bochner BS, Dang CV. Association of the decreased expression of alpha3 beta 1 integrin with the altered cell: environmental interactions and enhanced soft agar cloning ability of c-myc overexpressing small cell cancer cells. Cancer Res 58: 5537–5545, 1998. - PubMed
-
- Blystone SD Integrating an integrin: a direct route to actin. Biochim Biophys Acta 1692: 47–54, 2004. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

