Pleural mesothelial cell transformation into myofibroblasts and haptotactic migration in response to TGF-beta1 in vitro
- PMID: 19411308
- PMCID: PMC2711818
- DOI: 10.1152/ajplung.90587.2008
Pleural mesothelial cell transformation into myofibroblasts and haptotactic migration in response to TGF-beta1 in vitro
Abstract
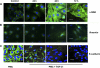
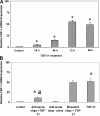
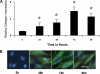
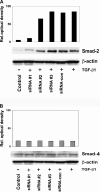
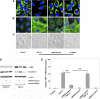
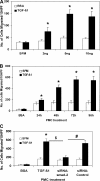
Idiopathic pulmonary fibrosis (IPF) is a disease of unknown etiology characterized by the development of subpleural foci of myofibroblasts that contribute to the exuberant fibrosis noted in the pulmonary parenchyma. Pleural mesothelial cells (PMC) are metabolically dynamic cells that cover the lung and chest wall as a monolayer and are in intimate proximity to the underlying lung parenchyma. The precise role of PMC in the pathogenesis of pulmonary parenchymal fibrosis remains to be identified. Transforming growth factor (TGF)-beta1, a cytokine known for its capacity to induce proliferative and transformative changes in lung cells, is found in significantly higher quantities in the lungs of patients with IPF. High levels of TGF-beta1 in the subpleural milieu may play a key role in the transition of normal PMC to myofibroblasts. Here we demonstrate that PMC activated by TGF-beta1 undergo epithelial-mesenchymal transition (EMT) and respond with haptotactic migration to a gradient of TGF-beta1 and that the transition of PMC to myofibroblasts is dependent on smad-2 signaling. The EMT of PMC was marked by upregulation of alpha-smooth muscle actin (alpha-SMA), fibroblast specific protein-1 (FSP-1), and collagen type I expression. Cytokeratin-8 and E-cadherin expression decreased whereas vimentin remained unchanged over time in transforming PMC. Knockdown of smad-2 gene by silencing small interfering RNA significantly suppressed the transition of PMC to myofibroblasts and significantly inhibited the PMC haptotaxis. We conclude that PMC undergo EMT when exposed to TGF-beta1, involving smad-2 signaling, and PMC may be a possible source of myofibroblasts in IPF.
Figures



References
-
- Abe R, Donnelly SC, Peng T, Bucala R, Metz CN. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol 166: 7556–7562, 2001. - PubMed
-
- Antony VB Immunological mechanisms in pleural disease. Eur Respir J 21: 539–544, 2003. - PubMed
-
- Aroeira LS, Aguilera A, Selgas R, Ramirez-Huesca M, Perez-Lozano ML, Cirugeda A, Bajo MA, del Peso G, Sanchez-Tomero JA, Jimenez-Heffernan JA, Lopez-Cabrera M. Mesenchymal conversion of mesothelial cells as a mechanism responsible for high solute transport rate in peritoneal dialysis: role of vascular endothelial growth factor. Am J Kidney Dis 46: 938–948, 2005. - PubMed
-
- Bonniaud P, Kolb M, Galt T, Robertson J, Robbins C, Stampfli M, Lavery C, Margetts PJ, Roberts AB, Gauldie J. Smad3 null mice develop airspace enlargement and are resistant to TGF-beta-mediated pulmonary fibrosis. J Immunol 173: 2099–2108, 2004. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

