Response of Listeria monocytogenes to Disinfection Stress at the Single-Cell and Population Levels as Monitored by Intracellular pH measurements and viable-cell counts
- PMID: 19411424
- PMCID: PMC2704842
- DOI: 10.1128/AEM.02625-08
Response of Listeria monocytogenes to Disinfection Stress at the Single-Cell and Population Levels as Monitored by Intracellular pH measurements and viable-cell counts
Abstract
Listeria monocytogenes has a remarkable ability to survive and persist in food production environments. The purpose of the present study was to determine if cells in a population of L. monocytogenes differ in sensitivity to disinfection agents as this could be a factor explaining persistence of the bacterium. In situ analyses of Listeria monocytogenes single cells were performed during exposure to different concentrations of the disinfectant Incimaxx DES to study a possible population subdivision. Bacterial survival was quantified with plate counting and disinfection stress at the single-cell level by measuring intracellular pH (pH(i)) over time by fluorescence ratio imaging microscopy. pH(i) values were initially 7 to 7.5 and decreased in both attached and planktonic L. monocytogenes cells during exposure to sublethal and lethal concentrations of Incimaxx DES. The response of the bacterial population was homogenous; hence, subpopulations were not detected. However, pregrowth with NaCl protected the planktonic bacterial cells during disinfection with Incimaxx (0.0015%) since pH(i) was higher (6 to 6.5) for the bacterial population pregrown with NaCl than for cells grown without NaCl (pH(i) 5 to 5.5) (P < 0.05). The protective effect of NaCl was reflected by viable-cell counts at a higher concentration of Incimaxx (0.0031%), where the salt-grown population survived better than the population grown without NaCl (P < 0.05). NaCl protected attached cells through drying but not during disinfection. This study indicates that a population of L. monocytogenes cells, whether planktonic or attached, is homogenous with respect to sensitivity to an acidic disinfectant studied on the single-cell level. Hence a major subpopulation more tolerant to disinfectants, and hence more persistent, does not appear to be present.
Figures


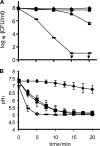
 ) Incimaxx DES. The viable-cell counts are averages of duplicate determinations. Error bars are based on standard deviations from the duplicate determinations. pHis are averages based on at least 40 single cells from Fig. 2, and error bars indicate population variation. Arrows indicate that CFU were under the detection limit in the plating assay (10 CFU/ml). The results are representative of two independent experiments.
) Incimaxx DES. The viable-cell counts are averages of duplicate determinations. Error bars are based on standard deviations from the duplicate determinations. pHis are averages based on at least 40 single cells from Fig. 2, and error bars indicate population variation. Arrows indicate that CFU were under the detection limit in the plating assay (10 CFU/ml). The results are representative of two independent experiments.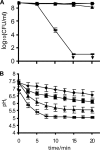
 ) Incimaxx DES. The viable-cell counts are averages of duplicate determinations. Error bars are based on standard deviations from the duplicate determinations. pHis are averages based on at least 40 single cells, and error bars indicate population variation. Arrows indicate that CFU were under the detection limit in the plating assay (10 CFU/ml). The results are representative of two independent experiments.
) Incimaxx DES. The viable-cell counts are averages of duplicate determinations. Error bars are based on standard deviations from the duplicate determinations. pHis are averages based on at least 40 single cells, and error bars indicate population variation. Arrows indicate that CFU were under the detection limit in the plating assay (10 CFU/ml). The results are representative of two independent experiments.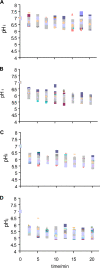

 ) Incimaxx DES. The viable-cell counts are averages of triplicate determinations. Error bars are based on standard deviations from the triplicate determinations. pHis are averages based on at least 40 single cells, and error bars indicate population variation. The results are representative of two independent experiments.
) Incimaxx DES. The viable-cell counts are averages of triplicate determinations. Error bars are based on standard deviations from the triplicate determinations. pHis are averages based on at least 40 single cells, and error bars indicate population variation. The results are representative of two independent experiments.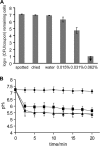
 ) Incimaxx DES. The viable-cell counts are averages of triplicate determinations. Error bars are based on standard deviations from the triplicate determinations. pHis are averages based on at least 40 single cells, and error bars indicate population variation. Arrows indicate that CFU were under the detection limit in the plating assay (100 CFU/coupon). The results are representative of two independent experiments.
) Incimaxx DES. The viable-cell counts are averages of triplicate determinations. Error bars are based on standard deviations from the triplicate determinations. pHis are averages based on at least 40 single cells, and error bars indicate population variation. Arrows indicate that CFU were under the detection limit in the plating assay (100 CFU/coupon). The results are representative of two independent experiments.References
-
- Aase, B., G. Sundheim, S. Langsrud, and L. M. Rørvik. 2000. Occurrence of and a possible mechanism for resistance to a quaternary ammonium compound in Listeria monocytogenes. Int. J. Food Microbiol. 62:57-63. - PubMed
-
- Balaban, N. Q., J. Merrin, R. Chait, L. Kowalik, and S. Leibler. 2004. Bacterial persistence as a phenotypic switch. Science 305:1622-1625. - PubMed
-
- Chasseignaux, E., M.-T. Toquin, C. Ragimbeau, G. Salvat, P. Colin, and G. Ermel. 2001. Molecular epidemiology of Listeria monocytogenes isolates collected from the environment, raw meat and raw products in two poultry- and pork-processing plants. J. Appl. Microbiol. 91:888-899. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

