GATA-1 associates with and inhibits p53
- PMID: 19411634
- PMCID: PMC2710945
- DOI: 10.1182/blood-2008-10-180489
GATA-1 associates with and inhibits p53
Abstract
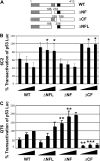
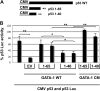
In addition to orchestrating the expression of all erythroid-specific genes, GATA-1 controls the growth, differentiation, and survival of the erythroid lineage through the regulation of genes that manipulate the cell cycle and apoptosis. The stages of mammalian erythropoiesis include global gene inactivation, nuclear condensation, and enucleation to yield circulating erythrocytes, and some of the genes whose expression are altered by GATA-1 during this process are members of the p53 pathway. In this study, we demonstrate a specific in vitro interaction between the transactivation domain of p53 (p53TAD) and a segment of the GATA-1 DNA-binding domain that includes the carboxyl-terminal zinc-finger domain. We also show by immunoprecipitation that the native GATA-1 and p53 interact in erythroid cells and that activation of p53-responsive promoters in an erythroid cell line can be inhibited by the overexpression of GATA-1. Mutational analysis reveals that GATA-1 inhibition of p53 minimally requires the segment of the GATA-1 DNA-binding domain that interacts with p53TAD. This inhibition is reciprocal, as the activation of a GATA-1-responsive promoter can be inhibited by p53. Based on these findings, we conclude that inhibition of the p53 pathway by GATA-1 may be essential for erythroid cell development and survival.
Figures





References
-
- Tsai SF, Martin DI, Zon LI, D'Andrea AD, Wong GG, Orkin SH. Cloning of cDNA for the major DNA-binding protein of the erythroid lineage through expression in mammalian cells. Nature. 1989;339:446–451. - PubMed
-
- Pevny L, Simon MC, Robertson E, et al. Erythroid differentiation in chimeric mice blocked by a targeted mutation in the gene for transcription factor GATA-1. Nature. 1991;349:257–260. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

