Protein phosphatase 2Cm is a critical regulator of branched-chain amino acid catabolism in mice and cultured cells
- PMID: 19411760
- PMCID: PMC2689111
- DOI: 10.1172/JCI38151
Protein phosphatase 2Cm is a critical regulator of branched-chain amino acid catabolism in mice and cultured cells
Abstract
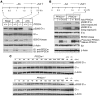
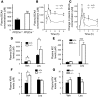
The branched-chain amino acids (BCAA) are essential amino acids required for protein homeostasis, energy balance, and nutrient signaling. In individuals with deficiencies in BCAA, these amino acids can be preserved through inhibition of the branched-chain-alpha-ketoacid dehydrogenase (BCKD) complex, the rate-limiting step in their metabolism. BCKD is inhibited by phosphorylation of its E1alpha subunit at Ser293, which is catalyzed by BCKD kinase. During BCAA excess, phosphorylated Ser293 (pSer293) becomes dephosphorylated through the concerted inhibition of BCKD kinase and the activity of an unknown intramitochondrial phosphatase. Using unbiased, proteomic approaches, we have found that a mitochondrial-targeted phosphatase, PP2Cm, specifically binds the BCKD complex and induces dephosphorylation of Ser293 in the presence of BCKD substrates. Loss of PP2Cm completely abolished substrate-induced E1alpha dephosphorylation both in vitro and in vivo. PP2Cm-deficient mice exhibited BCAA catabolic defects and a metabolic phenotype similar to the intermittent or intermediate types of human maple syrup urine disease (MSUD), a hereditary disorder caused by defects in BCKD activity. These results indicate that PP2Cm is the endogenous BCKD phosphatase required for nutrient-mediated regulation of BCKD activity and suggest that defects in PP2Cm may be responsible for a subset of human MSUD.
Figures


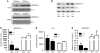


References
-
- Hutson S.M., Sweatt A.J., Lanoue K.F. Branched-chain [corrected] amino acid metabolism: implications for establishing safe intakes. J. Nutr. 2005;135:1557S–1564S. - PubMed
-
- Layman D.K., Walker D.A. Potential importance of leucine in treatment of obesity and the metabolic syndrome. J. Nutr. 2006;136:319S–323S. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL080111/HL/NHLBI NIH HHS/United States
- P01 HL080111/HL/NHLBI NIH HHS/United States
- R01 DK053843/DK/NIDDK NIH HHS/United States
- DK053843/DK/NIDDK NIH HHS/United States
- R21 HL096041/HL/NHLBI NIH HHS/United States
- HL70079/HL/NHLBI NIH HHS/United States
- DK062880/DK/NIDDK NIH HHS/United States
- HL62311/HL/NHLBI NIH HHS/United States
- R01 HL062311/HL/NHLBI NIH HHS/United States
- R01 HL087132/HL/NHLBI NIH HHS/United States
- S10 RR022371/RR/NCRR NIH HHS/United States
- HL087132/HL/NHLBI NIH HHS/United States
- RR 022371/RR/NCRR NIH HHS/United States
- R01 DK062880/DK/NIDDK NIH HHS/United States
- R01 HL070079/HL/NHLBI NIH HHS/United States
- HLP01-008111/PHS HHS/United States
- R01 HL088975/HL/NHLBI NIH HHS/United States
- R01 HL077440/HL/NHLBI NIH HHS/United States

