High-throughput ethomics in large groups of Drosophila
- PMID: 19412169
- PMCID: PMC2734963
- DOI: 10.1038/nmeth.1328
High-throughput ethomics in large groups of Drosophila
Abstract
We present a camera-based method for automatically quantifying the individual and social behaviors of fruit flies, Drosophila melanogaster, interacting in a planar arena. Our system includes machine-vision algorithms that accurately track many individuals without swapping identities and classification algorithms that detect behaviors. The data may be represented as an ethogram that plots the time course of behaviors exhibited by each fly or as a vector that concisely captures the statistical properties of all behaviors displayed in a given period. We found that behavioral differences between individuals were consistent over time and were sufficient to accurately predict gender and genotype. In addition, we found that the relative positions of flies during social interactions vary according to gender, genotype and social environment. We expect that our software, which permits high-throughput screening, will complement existing molecular methods available in Drosophila, facilitating new investigations into the genetic and cellular basis of behavior.
Figures

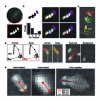

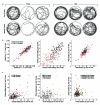

Comment in
-
The ethomics era?Nat Methods. 2009 Jun;6(6):413-4. doi: 10.1038/nmeth0609-413. Nat Methods. 2009. PMID: 19478800 No abstract available.
References
-
- Wolf FW, Heberlein U. Invertebrate models of drug abuse. Journal of Neurobiology. 2003;54:161–178. - PubMed
-
- Guarnieri D, Heberlein U. Drosophila melanogaster, a genetic model system for alcohol research. International Review of Neurobiology. 2003;54:199–228. - PubMed
-
- Hoyer SC, et al. Octopamine in Male Aggression of Drosophila. Current Biology. 2008 - PubMed
-
- Ho K, Sehgal A. Drosophila melanogaster: An insect model for fundamental studies of sleep. Methods Enzymol. 2005;393:772–793. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

