The defective prophage pool of Escherichia coli O157: prophage-prophage interactions potentiate horizontal transfer of virulence determinants
- PMID: 19412337
- PMCID: PMC2669165
- DOI: 10.1371/journal.ppat.1000408
The defective prophage pool of Escherichia coli O157: prophage-prophage interactions potentiate horizontal transfer of virulence determinants
Abstract
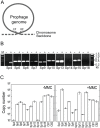
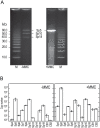
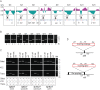
Bacteriophages are major genetic factors promoting horizontal gene transfer (HGT) between bacteria. Their roles in dynamic bacterial genome evolution have been increasingly highlighted by the fact that many sequenced bacterial genomes contain multiple prophages carrying a wide range of genes. Enterohemorrhagic Escherichia coli O157 is the most striking case. A sequenced strain (O157 Sakai) possesses 18 prophages (Sp1-Sp18) that encode numerous genes related to O157 virulence, including those for two potent cytotoxins, Shiga toxins (Stx) 1 and 2. However, most of these prophages appeared to contain multiple genetic defects. To understand whether these defective prophages have the potential to act as mobile genetic elements to spread virulence determinants, we looked closely at the Sp1-Sp18 sequences, defined the genetic defects of each Sp, and then systematically analyzed all Sps for their biological activities. We show that many of the defective prophages, including the Stx1 phage, are inducible and released from O157 cells as particulate DNA. In fact, some prophages can even be transferred to other E. coli strains. We also show that new Stx1 phages are generated by recombination between the Stx1 and Stx2 phage genomes. The results indicate that these defective prophages are not simply genetic remnants generated in the course of O157 evolution, but rather genetic elements with a high potential for disseminating virulence-related genes and other genetic traits to other bacteria. We speculate that recombination and various other types of inter-prophage interactions in the O157 prophage pool potentiate such activities. Our data provide new insights into the potential activities of the defective prophages embedded in bacterial genomes and lead to the formulation of a novel concept of inter-prophage interactions in defective prophage communities.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

