Helicobacter pylori usurps cell polarity to turn the cell surface into a replicative niche
- PMID: 19412339
- PMCID: PMC2669173
- DOI: 10.1371/journal.ppat.1000407
Helicobacter pylori usurps cell polarity to turn the cell surface into a replicative niche
Abstract
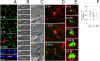
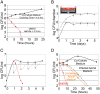
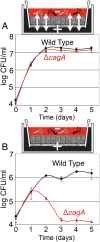
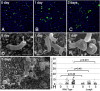
Helicobacter pylori (Hp) intimately interacts with the gastric epithelial surface and translocates the virulence factor CagA into host cells in a contact-dependent manner. To study how Hp benefits from interacting with the cell surface, we developed live-cell microscopy methods to follow the fate of individual bacteria on the cell surface and find that Hp is able to replicate and form microcolonies directly over the intercellular junctions. On polarized epithelia, Hp is able to grow directly on the apical cell surface in conditions that do not support the growth of free-swimming bacteria. In contrast, mutants in CagA delivery are defective in colonization of the apical cell surface. Hp perturbs the polarized epithelium in a highly localized manner, since wild-type Hp does not rescue the growth defect of the CagA-deficient mutants upon co-infection. CagA's ability to disrupt host cell polarity is a key factor in enabling colonization of the apical cell surface by Hp, as disruption of the atypical protein kinase C/Par1b polarity pathway leads to rescue of the mutant growth defect during apical infection, and CagA-deficient mutants are able to colonize the polarized epithelium when given access to the basolateral cell surface. Our study establishes the cell surface as a replicative niche and the importance of CagA and its effects on host cell polarity for this purpose.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Hendrixson DR, St Geme JW., 3rd The Haemophilus influenzae Hap serine protease promotes adherence and microcolony formation, potentiated by a soluble host protein. Mol Cell. 1998;2:841–850. - PubMed
-
- Phillips AD, Frankel G. Intimin-mediated tissue specificity in enteropathogenic Escherichia coli interaction with human intestinal organ cultures. J Infect Dis. 2000;181:1496–1500. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

