Comparison of T cell receptor-induced proximal signaling and downstream functions in immortalized and primary T cells
- PMID: 19412549
- PMCID: PMC2673025
- DOI: 10.1371/journal.pone.0005430
Comparison of T cell receptor-induced proximal signaling and downstream functions in immortalized and primary T cells
Abstract
Background: Human T cells play an important role in pathogen clearance, but their aberrant activation is also linked to numerous diseases. T cells are activated by the concurrent induction of the T cell receptor (TCR) and one or more costimulatory receptors. The characterization of signaling pathways induced by TCR and/or costimulatory receptor activation is critical, since these pathways are excellent targets for novel therapies for human disease. Although studies using human T cell lines have provided substantial insight into these signaling pathways, no comprehensive, direct comparison of these cell lines to activated peripheral blood T cells (APBTs) has been performed to validate their usefulness as a model of primary T cells.
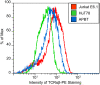
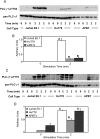
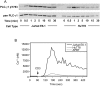
Methodology/principal findings: We used quantitative biochemical techniques to compare the activation of two widely used human T cell lines, Jurkat E6.1 and HuT78 T cells, to APBTs. We found that HuT78 cells were similar to APBTs in proximal TCR-mediated signaling events. In contrast, Jurkat E6.1 cells had significantly increased site-specific phosphorylation of Pyk2, PLCgamma1, Vav1, and Erk1/Erk2 and substantially more Ca2+ flux compared to HuT78 cells and APBTs. In part, these effects appear to be due to an overexpression of Itk in Jurkat E6.1 cells compared to HuT78 cells and APBTs. Both cell lines differ from APBTs in the expression and function of costimulatory receptors and in the range of cytokines and chemokines released upon TCR and costimulatory receptor activation.
Conclusions/significance: Both Jurkat E6.1 and HuT78 T cells had distinct similarities and differences compared to APBTs. Both cell lines have advantages and disadvantages, which must be taken into account when choosing them as a model T cell line.
Conflict of interest statement
Figures








References
-
- Nel AE. T-cell activation through the antigen receptor. Part 1: signaling components, signaling pathways, and signal integration at the T-cell antigen receptor synapse. J Allergy Clin Immunol. 2002;109:758–770. - PubMed
-
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. - PubMed
-
- Lechler RI, Sykes M, Thomson AW, Turka LA. Organ transplantation–how much of the promise has been realized? Nat Med. 2005;11:605–613. - PubMed
-
- Monaco C, Andreakos E, Kiriakidis S, Feldmann M, Paleolog E. T-cell-mediated signalling in immune, inflammatory and angiogenic processes: the cascade of events leading to inflammatory diseases. Curr Drug Targets Inflamm Allergy. 2004;3:35–42. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

