Hippocampal injury, atrophy, synaptic reorganization, and epileptogenesis after perforant pathway stimulation-induced status epilepticus in the mouse
- PMID: 19412934
- PMCID: PMC2705826
- DOI: 10.1002/cne.22059
Hippocampal injury, atrophy, synaptic reorganization, and epileptogenesis after perforant pathway stimulation-induced status epilepticus in the mouse
Abstract
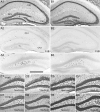
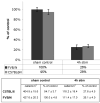
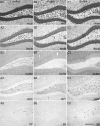
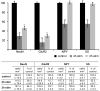
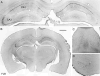
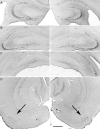
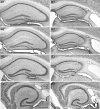
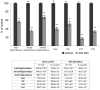
Prolonged dentate granule cell discharges produce hippocampal injury and chronic epilepsy in rats. In preparing to study this epileptogenic process in genetically altered mice, we determined whether the background strain used to generate most genetically altered mice, the C57BL/6 mouse, is vulnerable to stimulation-induced seizure-induced injury. This was necessary because C57BL/6 mice are reportedly resistant to the neurotoxic effects of kainate-induced seizures, which we hypothesized to be related to strain differences in kainate's effects, rather than genetic differences in intrinsic neuronal vulnerability. Bilateral perforant pathway stimulation-induced granule cell discharge for 4 hours under urethane anesthesia produced degeneration of glutamate receptor subunit 2 (GluR2)-positive hilar mossy cells and peptide-containing interneurons in both FVB/N (kainate-vulnerable) and C57BL/6 (kainate-resistant) mice, indicating no strain differences in neuronal vulnerability to seizure activity. Granule cell discharge for 2 hours in C57BL/6 mice destroyed most GluR2-positive dentate hilar mossy cells, but not peptide-containing hilar interneurons, indicating that mossy cells are the neurons most vulnerable to this insult. Stimulation for 24 hours caused extensive hippocampal neuron loss and injury to the septum and entorhinal cortex, but no other detectable damage. Mice stimulated for 24 hours developed hippocampal sclerosis, granule cell mossy fiber sprouting, and chronic epilepsy, but not the granule cell layer hypertrophy (granule cell dispersion) produced by intrahippocampal kainate. These results demonstrate that perforant pathway stimulation in mice reliably reproduces the defining features of human mesial temporal lobe epilepsy with hippocampal sclerosis. Experimental studies in transgenic or knockout mice are feasible if electrical stimulation is used to produce controlled epileptogenic insults.
Copyright 2009 Wiley-Liss, Inc.
Figures
Similar articles
-
Recurrent mossy fiber pathway in rat dentate gyrus: synaptic currents evoked in presence and absence of seizure-induced growth.J Neurophysiol. 1999 Apr;81(4):1645-60. doi: 10.1152/jn.1999.81.4.1645. J Neurophysiol. 1999. PMID: 10200201
-
Recurrent seizures and hippocampal sclerosis following intrahippocampal kainate injection in adult mice: electroencephalography, histopathology and synaptic reorganization similar to mesial temporal lobe epilepsy.Neuroscience. 1999 Mar;89(3):717-29. doi: 10.1016/s0306-4522(98)00401-1. Neuroscience. 1999. PMID: 10199607
-
Abnormal responses to perforant path stimulation in the dentate gyrus of slices from rats with kainate-induced epilepsy and mossy fiber reorganization.Epilepsy Res. 1999 Aug;36(1):31-42. doi: 10.1016/s0920-1211(99)00022-4. Epilepsy Res. 1999. PMID: 10463848
-
The functional organization of the hippocampal dentate gyrus and its relevance to the pathogenesis of temporal lobe epilepsy.Ann Neurol. 1994 Jun;35(6):640-54. doi: 10.1002/ana.410350604. Ann Neurol. 1994. PMID: 8210220 Review.
-
Temporal Lobe Epileptogenesis: A Focus on Etiology, Neuron Loss, the Latent Period, and Dentate Granule Cell Disinhibition.In: Noebels JL, Avoli M, Rogawski MA, Vezzani A, Delgado-Escueta AV, editors. Jasper's Basic Mechanisms of the Epilepsies. 5th edition. New York: Oxford University Press; 2024. Chapter 24. In: Noebels JL, Avoli M, Rogawski MA, Vezzani A, Delgado-Escueta AV, editors. Jasper's Basic Mechanisms of the Epilepsies. 5th edition. New York: Oxford University Press; 2024. Chapter 24. PMID: 39637110 Free Books & Documents. Review.
Cited by
-
Removing entorhinal cortex input to the dentate gyrus does not impede low frequency oscillations, an EEG-biomarker of hippocampal epileptogenesis.Sci Rep. 2016 May 10;6:25660. doi: 10.1038/srep25660. Sci Rep. 2016. PMID: 27160925 Free PMC article.
-
A companion to the preclinical common data elements for pharmacologic studies in animal models of seizures and epilepsy. A Report of the TASK3 Pharmacology Working Group of the ILAE/AES Joint Translational Task Force.Epilepsia Open. 2018 Sep 15;3(Suppl Suppl 1):53-68. doi: 10.1002/epi4.12254. eCollection 2018 Nov. Epilepsia Open. 2018. PMID: 30450485 Free PMC article.
-
Stem Cell Therapy for Post-Traumatic Stress Disorder: A Novel Therapeutic Approach.Diseases. 2021 Oct 29;9(4):77. doi: 10.3390/diseases9040077. Diseases. 2021. PMID: 34842629 Free PMC article. Review.
-
Reorganization of supramammillary-hippocampal pathways in the rat pilocarpine model of temporal lobe epilepsy: evidence for axon terminal sprouting.Brain Struct Funct. 2015 Jul;220(4):2449-68. doi: 10.1007/s00429-014-0800-2. Epub 2014 Jun 3. Brain Struct Funct. 2015. PMID: 24889162 Free PMC article.
-
High-field magnetic resonance imaging of the human temporal lobe.Neuroimage Clin. 2015 Aug 1;9:58-68. doi: 10.1016/j.nicl.2015.07.005. eCollection 2015. Neuroimage Clin. 2015. PMID: 26413472 Free PMC article.
References
-
- Abercrombie M. Estimation of nuclear population from microtome sections. Anat Rec. 1946;94:239–247. - PubMed
-
- Andersen P, Holmqvist B, Voorhoeve PE. Entorhinal activation of dentate granule cells. Acta Physiol Scand. 1966;66:448–460. - PubMed
-
- Blümcke I, Suter B, Behle K, Kuhn R, Schramm J, Elger CE, Wiestler OD. Loss of hilar mossy cells in Ammon’s horn sclerosis. Epilepsia. 2000;41(Suppl 6):S174–S180. - PubMed
-
- Blümcke I, Zuschratter W, Schewe JC, Suter B, Lie AA, Riederer BM, Meyer B, Schramm J, Elger CE, Wiestler OD. Cellular pathology of hilar neurons in Ammon’s horn sclerosis. J Comp Neurol. 1999;414:437–453. - PubMed

