Differential effects of acute and repeat dosing with the H3 antagonist GSK189254 on the sleep-wake cycle and narcoleptic episodes in Ox-/- mice
- PMID: 19413575
- PMCID: PMC2697793
- DOI: 10.1111/j.1476-5381.2009.00205.x
Differential effects of acute and repeat dosing with the H3 antagonist GSK189254 on the sleep-wake cycle and narcoleptic episodes in Ox-/- mice
Abstract
Background and purpose: Histamine H3 receptor antagonists are currently being evaluated in clinical trials for a number of central nervous system disorders including narcolepsy. These agents can increase wakefulness (W) in cats and rodents following acute administration, but their effects after repeat dosing have not been reported previously.
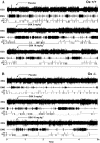
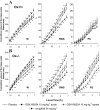
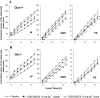
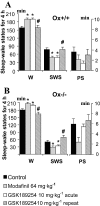
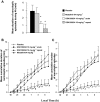
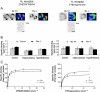
Experimental approach: EEG and EMG recordings were used to investigate the effects of acute and repeat administration of the novel H3 antagonist GSK189254 on the sleep-wake cycle in wild-type (Ox+/+) and orexin knockout (Ox-/-) mice, the latter being genetically susceptible to narcoleptic episodes. In addition, we investigated H3 and H1 receptor expression in this model using radioligand binding and autoradiography.
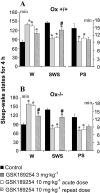
Key results: In Ox+/+ and Ox-/- mice, acute administration of GSK189254 (3 and 10 mg x kg(-1) p.o.) increased W and decreased slow wave and paradoxical sleep to a similar degree to modafinil (64 mg x kg(-1)), while it reduced narcoleptic episodes in Ox-/- mice. After twice daily dosing for 8 days, the effect of GSK189254 (10 mg x kg(-1)) on W in both Ox+/+ and Ox-/- mice was significantly reduced, while the effect on narcoleptic episodes in Ox-/- mice was significantly increased. Binding studies revealed no significant differences in H3 or H1 receptor expression between Ox+/+ and Ox-/- mice.
Conclusions and implications: These studies provide further evidence to support the potential use of H3 antagonists in the treatment of narcolepsy and excessive daytime sleepiness. Moreover, the differential effects observed on W and narcoleptic episodes following repeat dosing could have important implications in clinical studies.
Figures
References
-
- Arrang JM, Garbarg M, Schwartz JC. Auto-inhibition of brain histamine release mediated by a novel class (H3) of histamine receptor. Nature. 1983;302:832–837. - PubMed
-
- Arrang JM, Garbarg M, Lancelot JC, Lecomte JM, Pollard H, Robba M, et al. Potential interest in powerful and specific ligands for the histamine H3 receptor. Allerg Immunol (Paris) 1988;20:327–329. 331. - PubMed
-
- Billiard M, Bassetti C, Dauvilliers Y, Dolenc-Groselj L, Lammers GJ, Mayer G, et al. EFNS guidelines on management of narcolepsy. Eur J Neurol. 2006;13:1035–1048. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

