The spleen plays a central role in primary humoral alloimmunization to transfused mHEL red blood cells
- PMID: 19413728
- PMCID: PMC3568957
- DOI: 10.1111/j.1537-2995.2009.02200.x
The spleen plays a central role in primary humoral alloimmunization to transfused mHEL red blood cells
Abstract
Background: Several differences exist between antigens on transfused red blood cells (RBCs) and other immunogens, including anatomical compartmentalization. Whereas antigens from microbial pathogens and solid organ transplants drain into local lymph nodes, circulating RBCs remain segregated in the peripheral circulation, where they are consumed by antigen-presenting cells (APCs) in the spleen and liver. Accordingly, it was hypothesized that the splenic APCs play a central role in primary alloimmunization to transfused RBCs.
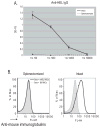
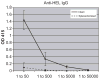
Study design and methods: Recipient mice were splenectomized and transfused with transgenic RBCs expressing the membrane-bound hen egg lysozyme (mHEL) model RBC antigen. In some experiments, mHEL-specific CD4+ T cells were adoptively transferred into recipient mice to allow investigation of helper T-cell responses. Unmanipulated or sham-splenectomized mice served as controls. Recombinant murine cytomegalovirus expressing mHEL (mHEL-MCMV) was used as a control non-RBC immunogen. Humoral responses were measured by mHEL-specific enzyme-linked immunosorbent assay and flow cytometric–based RBC cross-match.
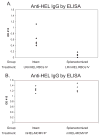
Results: Control animals synthesized detectable anti-HEL immunoglobulin (Ig)G after a single mHEL RBC transfusion. mHEL-specific CD4+ T cells underwent robust expansion, and adoptive transfer of CD4+ T cells resulted in a 1000-fold increase in anti-HEL IgG. In contrast, minimal anti-HEL IgG was detectable in splenectomized mice, mHEL-specific CD4+ T cells did not proliferate, and adoptive transfer did not increase anti-HEL IgG. However, anti-HEL IgG response after exposure to mHEL-MCMV was equivalent in control and splenectomized mice.
Discussion: Together, these findings illustrate the distinct properties of transfused RBCs as immunologic stimuli, with the spleen playing a critical role in primary RBC alloimmunization at the level of CD4+ T-cell activation.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures

References
-
- Blood banking and transfusion medicine. 2. Philadelphia (PA): Churchill Livingstone, Elsevier; 2007.
-
- Heddle NM, Soutar RL, O’Hoski PL, Singer J, McBride JA, Ali MA, Kelton JG. A prospective study to determine the frequency and clinical significance of alloimmunization post-transfusion. Br J Haematol. 1995;91:1000–5. - PubMed
-
- Hoeltge GA, Domen RE, Rybicki LA, Schaffer PA. Multiple red cell transfusions and alloimmunization. Experience with 6996 antibodies detected in a total of 159,262 patients from 1985 to 1993. Arch Pathol Lab Med. 1995;119:42–5. - PubMed
-
- Seyfried H, Walewska I. Analysis of immune response to red blood cell antigens in multitransfused patients with different diseases. Mat Med Pol. 1990;22:21–5. - PubMed
-
- Hendrickson JE, Chadwick TE, Roback JD, Hillyer CD, Zimring JC. Inflammation enhances consumption and presentation of transfused RBC antigens by dendritic cells. Blood. 2007;110:2736–43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

